Introduction: Why Analytical Method Development Matters for FDA & Health Canada
Analytical method development is one of the most important steps in drug development and regulatory filing. Whether you’re submitting your application to the FDA in the United States or Health Canada, your analytical methods must be accurate, reliable, and validated. These methods confirm the quality, identity, strength, and purity of your pharmaceutical product.
At ResolveMass Laboratories Inc., we specialize in Analytical Method Development in Montreal, Canada and United States (US) for both small molecules and biologics. Our team supports pharmaceutical and biotech companies with method design, validation, transfer, and documentation that meet ICH and regulatory standards.
👉 Explore Analytical Method Development Services
What Is Analytical Method Development?
Analytical method development is the process of designing lab-based tests that can accurately measure key properties of a drug substance or drug product. These include:
- Assay (active ingredient content)
- Impurity profiling
- Dissolution testing
- Identification and quantification
- Content uniformity
Once the method is developed, it must be validated to ensure it works consistently across different labs and instruments. This process is critical for FDA and Health Canada approval.
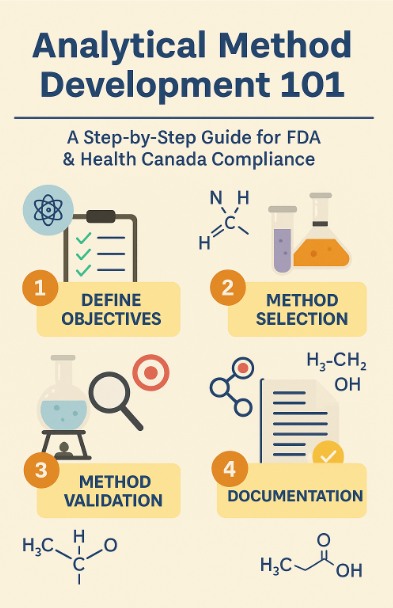
Step-by-Step Guide to Analytical Method Development
Step 1: Understanding the Drug Profile
The first step is to understand the nature of the drug, its solubility, stability, pKa, and chemical behavior. Knowing this helps determine the analytical technique to use—like HPLC, GC, LC-MS, UV, or NMR.
Step 2: Selecting the Right Technique
Depending on the target analyte, we choose the most suitable method:
- HPLC for quantifying active pharmaceutical ingredients
- LC-MS for detecting low-level impurities or degradation products
- UV-spectroscopy for simpler formulations
- NMR for structure confirmation (Check our NMR Services)
Step 3: Method Development and Optimization
In this phase, we work on:
- Mobile phase and solvent selection
- Column type and particle size
- Detector settings and wavelengths
- Flow rate and run time
We optimize all variables to get sharp, reproducible peaks and accurate results.
Step 4: Method Validation
Validation is done according to ICH Q2(R1) and includes the following parameters:
- Accuracy
- Precision
- Specificity
- Linearity
- Range
- Limit of Detection (LOD)
- Limit of Quantitation (LOQ)
- Robustness
We provide full validation reports ready for submission to FDA or Health Canada.
Step 5: Method Transfer or Verification
If the method is developed at ResolveMass, we help transfer it to your QC lab or manufacturing partner. We also perform verification if the method is adopted from literature or pharmacopeial sources.
📍 We specialize in Analytical Method Development in Montreal, Canada and United States (US), offering complete support from development to regulatory documentation.
Why Regulatory Authorities Care So Much
FDA and Health Canada want proof that your methods are reliable and reproducible. Poor analytical methods can lead to:
- Rejected filings (ANDA/NDA)
- Data deficiency letters
- Delays in approval
With ResolveMass, your methods are:
- Designed by experts
- Aligned with ICH and regulatory guidelines
- Backed by validated data
Case Study: Method Development for a Nasal Spray ANDA Submission
Client: Canadian generic company
Goal: Develop a stability-indicating HPLC method for a steroidal nasal spray formulation
Method Used:
- Reversed-phase HPLC
- UV detection at 254 nm
- Gradient elution with acetonitrile and buffer
Validation Highlights:
- Linearity: r² = 0.9992 (0.5–150 µg/mL)
- Precision: RSD < 1.5%
- LOD: 0.15 µg/mL
Outcome:
- Full method report submitted in ANDA
- Accepted by FDA with zero deficiencies
- Method transferred successfully to client’s in-house QC lab
Common Challenges We Help You Solve
- Mixed peak separation
- Less sensitivity in impurity detection
- Matrix interference
- Data reproducibility issues
With 10+ years of method development experience, ResolveMass ensures your methods are robust and regulatory ready
Conclusion
Analytical method development is not just a technical task—it is the foundation of your regulatory success. ResolveMass Laboratories provides expert-level Analytical Method Development in Montreal Canada and United States (US) with full regulatory alignment and technical reliability. Whether you’re developing methods for a new IND, ANDA, or Health Canada CTA, we make sure you’re submission-ready.
✅ Submit your method development inquiry
✅ Schedule a discovery call with our scientists
✅ Start your project with our analytical chemistry lab
Need expert support for your FDA or Health Canada filing? ResolveMass Laboratories Inc. is here to help with expert-level Analytical Method Development in Montreal, Canada and United States (US). Contact our team today via our contact form, or call us directly for consultation. We typically respond within 24 hours.
📍 Location: Montreal, Quebec, Canada
📧 Email: info@resolvemass.ca
🌐 Contact Page: https://resolvemass.ca/contact/
✅ References
- ICH Q2(R1) – Validation of Analytical Procedures: Text and Methodology. https://www.ich.org/page/quality-guidelines
- FDA Guidance: Analytical Procedures and Methods Validation for Drugs and Biologics. https://www.fda.gov/media/87801/download
- Health Canada: Guidance for Industry – Quality (Chemistry and Manufacturing) Guidance: New Drug Submissions. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents.html
The Role of Extractables and Leachables (E&L) in Carcinogenicity Risk
Introduction Extractables and Leachables Carcinogenicity Testing plays a vital role in protecting patients who rely…
Case Study: Forced Degradation Study of Gimeracil — Discovery and Structure Elucidation of Novel Impurities
Introduction Forced degradation studies are a cornerstone of modern pharmaceutical development, enabling scientists to intentionally…
Affordable Bioanalytical Services for Start-Up Biotech: What to Expect
INTRODUCTION Affordable bioanalytical services for start-up biotech companies provide the essential analytical support needed to…
Top Bioanalytical Services for Small & Large Molecule Quantification
Introduction Bioanalytical services for small & large molecule quantification are essential for ensuring precision, sensitivity,…
Why Choose ResolveMass Laboratories for Bioanalytical Services: A Service Overview
INTRODUCTION The short answer is: clients choose ResolveMass because this ResolveMass Bioanalytical Services Overview demonstrates…
Case Study: Forced Degradation and Impurity Profiling of Nelarabine — Structural Elucidation of Novel Degradation Products
Introduction: The Critical Role of Forced Degradation in Defining Nelarabine Stability Forced degradation testing continues…