Introduction
Nitrosamine testing in Apixaban has become a top priority for pharmaceutical manufacturers due to rising safety concerns and stricter regulations. Apixaban is a commonly prescribed blood thinner used to prevent strokes and treat blood clots. Because even small amounts of nitrosamines in medicines like Apixaban can be harmful, labs like ResolveMass Laboratories are stepping up to help companies stay compliant and keep their products safe.
At ResolveMass, we specialize in advanced nitrosamine testing for both active pharmaceutical ingredients (APIs) and finished products. Our testing methods are precise, reliable, and tailored specifically for drugs such as Apixaban. We support pharmaceutical companies in avoiding recalls, meeting global standards, and most importantly, protecting patient health.
Understanding the Structure of Apixaban and Its Nitrosation Risk
Apixaban is a direct oral anticoagulant that blocks Factor Xa, preventing blood clots. Its structure includes parts like a piperidine ring and aromatic linkages, which are common in many pharmaceutical compounds.
One key feature is the secondary amine in the piperidine ring. This group can potentially form nitrosamines when it reacts with nitrites. That’s why nitrosamine testing in Apixaban is necessary—not only for compliance but also for long-term product safety.
Synthetic Pathway of Apixaban and Its Relevance to Nitrosamine Formation
The synthesis of Apixaban involves multiple reaction steps, including amide bond formation and arylation, often utilizing intermediates that contain amine groups. These amines have the potential to interact with nitrosating agents, which makes the synthesis stage a key area of concern for nitrosamine formation. A clear understanding of the synthetic pathway is essential for developing effective impurity control measures and testing approaches.
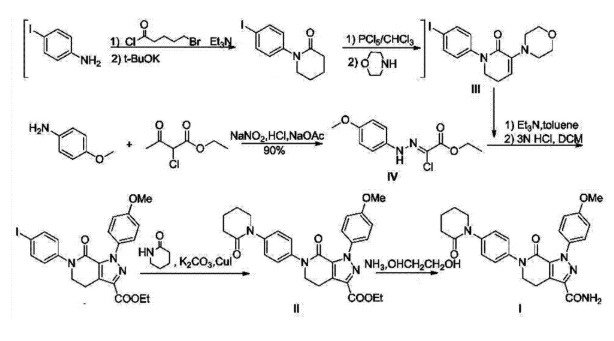
Gu, X., Xu, J., & Lu, M. (2014). Synthetic method of novel Apixaban (CN103896940A). CHEMFUTURE PHARMATECH (JIANGSU) Ltd. https://worldwide.espacenet.com/patent/search/family/048472163/publication/CN103896940A
Nitrosamine Risks in Apixaban Synthesis
The synthesis of Apixaban involves several amide bond-forming reactions and the introduction of a pyrazole-linked secondary amine, which presents a potential risk for nitrosamine formation. This secondary amine can undergo nitrosation when exposed to trace levels of nitrosating agents—such as sodium nitrite or nitrous acid—particularly under acidic conditions that may occur during coupling or purification steps.
A key impurity identified during synthesis is N-Nitroso Apixaban, also known in commercial terms as Nitroso Impurity 6 or N-Nitroso Apixaban Impurity B (molecular weight approximately 506.5). This compound forms when the secondary amine on the amino-pyridine ring is nitrosated, either before the final crystallization step or during intermediate purification.
If not effectively controlled, this impurity can carry over into the final active pharmaceutical ingredient (API). To prevent its formation, the following control measures are recommended:
- Avoid the use of reagents or solvents containing nitrites
- Maintain strict pH control during amide bond-forming steps
- Employ targeted LC-MS/MS methods for specific detection and monitoring of N-Nitroso Apixaban
For a more technical breakdown, consult our Nitrosamine CRO support page.
Nitrosamine Formation in Finished Apixaban Products
Apixaban can undergo nitrosation during formulation if exposed to trace nitrites from excipients like magnesium stearate or talc, especially under moist or acidic conditions. This may result in the formation of N-Nitroso Apixaban and related nitrosamine impurities.
Reported nitrosamines include:
- N-Nitroso Apixaban (Nitroso Impurity 6, MW ~506.5)
- Nitroso Apixaban Impurity 7 (MW ~505.5)
- Nitroso Apixaban Impurity 9 (MW ~507.5)
These impurities have been identified during stability testing and are closely monitored due to their potential genotoxicity.
To prevent formation:
- Use low-nitrite grade excipients
- Avoid high humidity and heat during processing
- Apply validated LC-MS/MS methods for detecting nitrosamines in the final product
These measures are essential to ensure nitrosamine levels remain within regulatory limits throughout the product’s shelf life.
For your product, access our nitrosamine risk assessment guide.
Key Challenges in Nitrosamine Testing for Apixaban
Apixaban’s structure and production process make nitrosamine testing more complicated than usual. Specific challenges include:
- Its chemical structure is prone to forming nitrosamines
- Some impurities appear over long storage periods
- Differences between batches can affect results
- Certain ingredients or steps may use secondary amines, which increase nitrosamine risk
Our team applies a comprehensive NDSRIs-focused workflow to detect both known and unknown nitrosamines, ensuring thorough impurity profiling.
Conclusion
Nitrosamine testing in Apixaban is now a critical step in the pharmaceutical manufacturing process. As regulations grow stricter, companies must take proactive steps to manage risks. With reliable support from ResolveMass Laboratories, you can feel confident that your Apixaban products meet every standard and are safe for patients.
We provide full services—from risk analysis to final lab reports—designed to help your business succeed in a tightly regulated market.
Explore more about:
- NDSRIs in Nitrosamine Testing
- Apixaban testing methodology
- Our Lab’s testing capability
- Risk Assessment support
📞 Contact ResolveMass Today
Frequently Asked Questions (FAQs)
Nitrosamines are chemical impurities that can potentially cause cancer when people are exposed to them over time. In drugs like Apixaban, these impurities may form during manufacturing or storage, especially when certain amine-based ingredients react with nitrosating agents. This risk makes it important to monitor and control their presence in pharmaceutical products.
The nitrosamines most commonly linked with Apixaban include NDMA (N-Nitrosodimethylamine), NDEA (N-Nitrosodiethylamine), and other nitrosamine drug substance-related impurities (NDSRIs). These impurities may result from specific chemicals or conditions used during synthesis, or from degradation over time if the product is not properly stored.
Yes, nitrosamine testing is now a mandatory requirement by regulatory agencies like the FDA, EMA, and Health Canada for medications such as Apixaban. Drug manufacturers must conduct risk assessments and confirmatory testing to ensure nitrosamine levels remain within safe limits. This helps protect patient health and ensures the product meets quality standards.
Our laboratory uses highly sensitive techniques like LC-MS/MS and GC-MS, which can detect nitrosamines in Apixaban at levels as low as 10 parts per billion (ppb) or even lower. These methods are validated and aligned with global regulatory expectations, ensuring precise and reliable results for both APIs and finished drug products.
ResolveMass is known for its scientific expertise, advanced equipment, and strong understanding of pharmaceutical regulations. We offer customized testing solutions for Apixaban and provide full support during audits or inspections. Our team ensures that results are both accurate and delivered on time, helping clients stay compliant and confident.
Typically, nitrosamine testing for Apixaban takes between 5 to 10 business days, depending on the complexity of the sample and the required method. We also offer expedited testing options for urgent projects. Our process includes method selection, sample analysis, and detailed reporting with clear and actionable data.
Yes, we provide complete risk assessment reports specifically for nitrosamines in Apixaban. These reports follow the latest FDA and EMA guidelines and include detailed evaluations of potential sources of contamination, testing results, and recommended preventive actions. This documentation is suitable for submission to regulatory authorities.
Yes, nitrosamines can form during the storage of Apixaban, especially if the product is exposed to heat, humidity, or unsuitable packaging materials. That’s why long-term stability testing is crucial to monitor impurity levels over time. Proper storage conditions and packaging design can greatly reduce this risk.
Yes, in addition to Apixaban, we offer nitrosamine testing services for other direct oral anticoagulants (DOACs) such as rivaroxaban, dabigatran, and edoxaban. Each of these drugs has its own synthesis and impurity profile, so we tailor our methods accordingly to ensure accurate and regulatory-compliant results.
Absolutely. We understand that different formulations, manufacturing methods, and excipients can affect nitrosamine risks. That’s why our testing approach is fully customized based on your product’s unique characteristics. We work closely with you to develop the right testing plan that fits your formulation and regulatory needs.
References
- Gu, X., Xu, J., & Lu, M. (2014). Synthetic method of novel Apixaban (CN103896940A). CHEMFUTURE PHARMATECH (JIANGSU) Ltd. https://worldwide.espacenet.com/patent/search/family/048472163/publication/CN103896940A
- Control of Nitrosamine Impurities in Human Drugs


