Nitrosamine contamination is a serious concern in many medicines today. Because of this, Nitrosamine Testing in Quetiapine has become a top priority for pharmaceutical companies. Quetiapine, a medication used to treat schizophrenia and bipolar disorder, is made using chemical steps that may create unwanted nitrosamine impurities. These can form both during the making of the active ingredient (API) and while preparing the final tablet. This article explains where the risks come from and how companies can stay safe and follow all rules.
With global health authorities tightening regulations, testing for nitrosamines is not just a safety measure but also a compliance requirement. Effective testing helps ensure the medicine remains safe for patients and maintains trust in pharmaceutical brands.
Understanding the Synthesis of Quetiapine
Quetiapine is produced using a few important chemical steps. It starts with a base compound called dibenzothiazepine and then goes through changes to add a side chain with a piperazine ring. In this process:
- Chemicals like ethoxyethanol help add the side chain.
- Solvents such as NMP (N-methylpyrrolidone) and DMA (dimethylacetamide) are often used.
- The process includes both alkylation and cyclization reactions.
These steps can lead to the formation of nitrosamines, especially when there are secondary or tertiary amines present. When these react with nitrosating agents (like nitrites), harmful N-nitroso compounds can form. Careful selection of solvents and reaction conditions helps reduce this risk.
Nitrosamine Risk Assessment in Quetiapine – API Synthesis
During the API synthesis, several materials and conditions can trigger nitrosamine formation. Nitrosamine Testing in Quetiapine should cover the following risk points:
- Piperazine structures in the API contain secondary amines that can be easily nitrosated.
- Use of solvents like DMA and NMP can lead to known nitrosamines such as NDMA and NMP-NO.
- Sodium nitrite, used in some steps, increases the chance of nitrosation.
Recycled solvents may carry over impurities from previous batches, and exposure to acid, air, or peroxide makes things worse. That’s why companies must regularly monitor for impurities like NPIP (N-nitroso-piperazine) and possible N-nitroso versions of quetiapine itself.
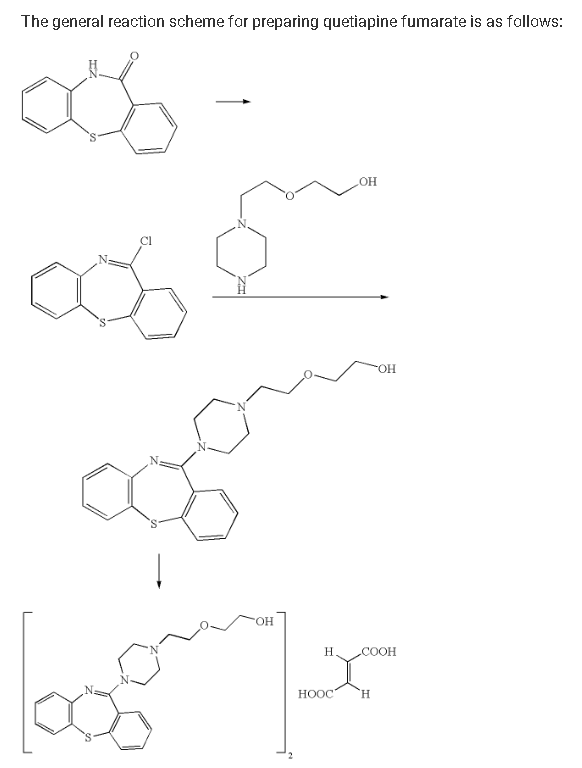
Kansal, V. K., Lal, K., Ahmad, S., & Leonov, D. (2010). Process for preparing quetiapine fumarate (U.S. Patent No. 7,687,622). U.S. Patent and Trademark Office. https://patents.google.com/patent/US7687622B2
Nitrosamine Testing in Quetiapine: Risks in Finished Tablets
Even after the API is made, the final tablet may still form nitrosamines under certain conditions. This can happen when the drug interacts with excipients or breaks down over time. Key risks include:
- The piperazine ring in Quetiapine can still react to form nitrosamines.
- Excipients such as sodium starch glycolate or microcrystalline cellulose may introduce trace nitrites.
- Moisture, heat, or light can cause breakdown of coating materials like HPMC or PVP, which might release amines.
To avoid this, forced degradation studies and long-term stability testing are essential. These tests help simulate real-life storage and check that nitrosamines stay below the safe limits.
Known and Potential Nitrosamine Impurities
Even though no nitrosamines are officially listed for Quetiapine yet, past research and chemical logic suggest that the following impurities might appear:
- N-Nitroso Quetiapine – Formed from the secondary amine in the API.
- NPIP (N-Nitroso-piperazine) – Comes from the piperazine side chain.
- NDMA – Linked to the use of DMA as a solvent.
- NMBA – May form during early steps of synthesis.
- NMP-NO – From the degradation of NMP.
- NEIPA – Could result from isopropyl amine contamination.
- NDEA – May come from tertiary amines breaking down.
It is important to map out where each of these might form and regularly test using proven methods like LC-MS/MS or GC-MS.
Summary Table: Where Nitrosamines May Appear in Quetiapine
| Production Stage | Likely Nitrosamines | Key Risk Factors |
|---|---|---|
| API Synthesis | NDMA, NPIP, NMP-NO, NMBA, NDEA | Solvents, piperazine, nitrites |
| Final Product | N-Nitroso Quetiapine, NEIPA, NPIP | Heat, moisture, excipients, degradation |
| Recycled Solvents | NDMA, NDEA | Cross-contamination, leftover nitrites |
| Oxidative Environments | NPIP, NMBA, other NDSRIs | Light, air, peroxide exposure |
Resources for Further Reading
- Nitrosamine Testing in Orphenadrine
- Nitrosamine Testing in Nizatidine
- Nitrosamine Testing in Metformin
- NDSRIs in Nitrosamine Testing
- Acceptable Intake of Nitrosamines
- Nitrosamine CRO Services
- Nitrosamine Risk Assessment Guide
- ResolveMass Nitrosamine Analysis Lab
📞 Need Help With Nitrosamine Testing in Quetiapine?
Get in touch with our experts today for full support on regulatory testing, impurity profiling, and safe formulation development.
Top Questions About Nitrosamine Testing in Quetiapine
The main impurity in quetiapine related to nitrosamines is N-nitroso quetiapine. This impurity can form during the manufacturing process or over time during storage. It is considered harmful because nitrosamines are known to be possible cancer-causing agents.
To test quetiapine, labs usually use HPLC (High-Performance Liquid Chromatography) to check purity and strength. For nitrosamine impurities, LC-MS/MS (Liquid Chromatography–Mass Spectrometry) or GC-MS is used. These methods help detect even tiny amounts of harmful substances.
Quetiapine Nitroso Impurity B is a nitrosamine-type impurity that may form from the piperazine part of the quetiapine molecule. It’s one of the NDSRI (nitrosamine drug substance-related impurities). It is closely monitored because of its potential health risks.
Lab tests often measure quetiapine levels in blood, especially in overdose or monitoring cases. Normal therapeutic levels usually range from 100 to 1000 ng/mL, but this can vary. Doctors use these values to check if the drug is working safely and correctly.
Impurity C in quetiapine is a known degradation product formed when the drug breaks down over time. It is listed in pharmacopeias and must be kept below certain limits during quality checks. Monitoring this impurity ensures the medicine remains safe and effective.
Quetiapine is considered high risk because its structure includes piperazine, which can form harmful nitrosamine impurities. Also, improper manufacturing or poor storage conditions can increase impurity levels. This makes strict testing and control very important.
Toxic levels of quetiapine are usually above 1000 ng/mL in the blood. In overdose cases, levels can go much higher and cause serious side effects like sleepiness, low blood pressure, or even coma. Immediate medical help is needed if overdose is suspected.
A safe dose of quetiapine depends on the patient and condition. For adults, the usual range is 150 mg to 800 mg per day, divided into smaller doses. Doctors start with a low dose and slowly increase it to avoid side effects and ensure safety.
References
- Kansal, V. K., Lal, K., Ahmad, S., & Leonov, D. (2010). Process for preparing quetiapine fumarate (U.S. Patent No. 7,687,622). U.S. Patent and Trademark Office. https://patents.google.com/patent/US7687622B2
- Control of Nitrosamine Impurities in Human Drugs
- Information about Nitrosamine Impurities in Medications


