Introduction
Nitrosamine Testing in Clarithromycin is now a crucial step for pharmaceutical manufacturers, driven by strict global regulatory requirements. Clarithromycin, a commonly prescribed macrolide antibiotic, can form nitrosamine impurities — such as Nitrosamine Drug Substance Related Impurities (NDSRIs) — during both API manufacturing and finished product preparation. Even very small amounts of these impurities are a concern because many nitrosamines are considered probable human carcinogens. Meeting the testing requirements set by agencies like Health Canada, the US FDA, and the EMA helps ensure that patients are not exposed to harmful substances over time. Manufacturers are also expected to include preventive measures in their production and storage processes to reduce nitrosamine risks.
For detailed guidance on nitrosamine impurity limits, visit:
- Nitrosamine Impurity Limits for Health Canada Submissions
- Nitrosamine Impurity Testing FAQ
- Nitrosamine Impurities in Pharmaceuticals
Understanding the Synthesis of Clarithromycin and Its Risks
Clarithromycin is made by modifying erythromycin A through several chemical steps, including O-methylation and oxime protection, followed by controlled deprotection stages. These steps often involve solvents like N,N-dimethylformamide (DMF) and specific catalysts. During this multi-step process, there are chances for nitrosamine formation, especially if secondary or tertiary amines, nitrites, or oxidizing agents are introduced. Even minor process deviations can allow these precursors to form or remain in the product. This is why regular monitoring using sensitive methods such as LC-MS/MS is now widely recommended to detect nitrosamines at extremely low levels.
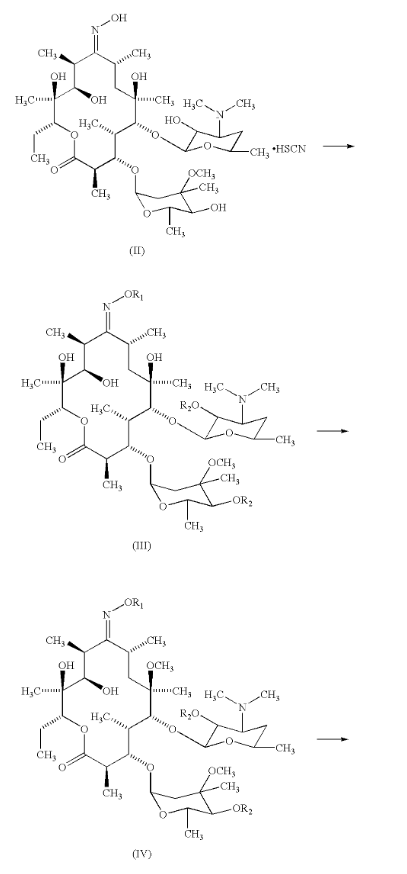
Rao, X., Ding, Z., Lou, H., Wu, J., Fang, Y., & Deng, B. (2012). Method of preparing clarithromycin (U.S. Patent No. 8,288,514). U.S. Patent and Trademark Office. https://patents.google.com/patent/US8288514B2
Nitrosamine Risk Assessment in Clarithromycin API Synthesis
Certain stages of Clarithromycin API production pose a higher risk of nitrosamine formation:
- DMF use: Degradation may release dimethylamine (DMA), which can react with nitrites to form N-nitrosodimethylamine (NDMA).
- DMA or NMP use: N-methylpyrrolidone (NMP) can degrade into amines that form N-nitrosomorpholine (NMOR).
- Catalysts: Some catalysts or phase transfer agents release DMA or diethylamine, leading to N-nitrosodiethylamine (NDEA).
- Oxime protection: Contaminated reagents during oxime formation can lead to nitrosooxime derivatives.
- Recycled solvents: Impurities from recycled DMF or DMA can reintroduce nitrosamines into the process.
A complete risk assessment should review raw materials, supplier quality, and in-process controls. Many manufacturers now use real-time monitoring to catch nitrosamine formation early and apply validated cleaning methods for equipment and solvents to reduce contamination risks.
Reference nitrosamine testing protocols:
- Nitrosamine CRO Support for Effective Risk Evaluation
- Nitrosamine Risk Assessment Guide for Your Drug Product
Nitrosamine Testing in Clarithromycin Finished Product
In the final dosage form, additional factors can contribute to nitrosamine presence:
- Nitrosatable amines in impurities: Degradation products containing secondary amines can react with nitrites.
- Nitrite-containing excipients: Certain stabilizers or lubricants may have trace nitrites.
- Degradation during storage: High humidity, heat, or acidic conditions can cause Clarithromycin to break down into amine-containing substances.
- Packaging contamination: In rare cases, nitrites from packaging materials may migrate into the product.
Routine stability testing, both accelerated and real-time, helps identify nitrosamine risks during storage. Using low-nitrite excipients and protective packaging can greatly reduce contamination chances. Quality risk management frameworks such as ICH Q9 (R1) are useful for designing effective storage and distribution controls.
Explore testing solutions:
Known Nitrosamine Impurities in Clarithromycin
Regulatory findings have identified several key nitrosamine contaminants in Clarithromycin:
- NDMA: Linked to DMF or DMA breakdown.
- NDEA: Associated with diethylamine-based catalysts.
- NMOR: Produced from NMP degradation.
- Nitrosooxime derivatives: Formed during oxime protection steps.
- Macrolide-specific NDSRIs: Unique nitrosamines requiring specialized testing methods.
Since NDSRIs are specific to macrolides, they require custom analytical methods for accurate detection. Advanced mass spectrometry techniques, especially those with isotope dilution, are highly effective for measuring these impurities at very low concentrations.
For more on NDSRIs:
Conclusion
Nitrosamine Testing in Clarithromycin is more than just meeting regulatory requirements — it’s about ensuring patient safety and maintaining product quality. Risks in API production include NDMA, NDEA, NMOR, and nitrosooxime formation, especially from solvent use, oxime reactions, and recycling processes. In the finished product, risks can arise from excipients, degradation pathways, and even packaging. Effective prevention includes strong supplier controls, validated analytical methods, and proper storage conditions. Expert laboratories like ResolveMass Laboratories offer the technical support needed to meet Health Canada, US FDA, and EMA standards, ensuring safety and compliance while protecting public health.
Contact ResolveMass Laboratories Inc.
Frequently Asked Questions (FAQs)
In Clarithromycin API, the most likely nitrosamines include N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), and N-nitrosomorpholine (NMOR). These can form during manufacturing from certain solvents, catalysts, or reaction conditions. Some macrolide-specific nitrosamines, called NDSRIs, may also appear due to Clarithromycin’s unique structure.
Direct use of nitrosating agents in Clarithromycin synthesis is uncommon. However, certain raw materials or contaminants, such as nitrites in reagents or water, can create nitrosating conditions. If these react with amines in the process, they may lead to nitrosamine formation even without intentional nitrosating chemicals.
N,N-Dimethylformamide (DMF) can break down into dimethylamine (DMA) during processing. DMA can easily react with nitrites to form NDMA, a harmful nitrosamine. This is why controlling DMF quality and monitoring for its degradation products is important in Clarithromycin manufacturing.
Yes. Nitrosamine Drug Substance Related Impurities (NDSRIs) are relevant because Clarithromycin, as a macrolide antibiotic, can form unique nitrosamines linked to its structure. These NDSRIs may not be detected by general nitrosamine tests, so specialized analytical methods are often needed.
Some excipients can contribute to nitrosamine formation if they contain trace nitrites. For example, certain binders, disintegrants, or stabilizers might carry small amounts of nitrites that react with amines in Clarithromycin, especially during storage under heat or humidity.
Yes. If recycled solvents are not thoroughly purified, they may carry pre-formed nitrosamines or their chemical precursors back into the process. This can lead to contamination in both the API and finished product stages of Clarithromycin manufacturing.
Acceptable nitrosamine limits are provided in guidelines from Health Canada, the U.S. Food and Drug Administration (FDA), and the European Medicines Agency (EMA). These agencies set strict daily intake limits for specific nitrosamines to ensure patient safety.
In Canada, specialized pharmaceutical testing labs such as ResolveMass Laboratories offer nitrosamine testing for Clarithromycin. They use advanced methods like LC-MS/MS to detect even trace amounts of impurities, ensuring compliance with regulatory standards.
References
- Rao, X., Ding, Z., Lou, H., Wu, J., Fang, Y., & Deng, B. (2012). Method of preparing clarithromycin (U.S. Patent No. 8,288,514). U.S. Patent and Trademark Office. https://patents.google.com/patent/US8288514B2
- U.S. Food and Drug Administration. (2024, September). Control of nitrosamine impurities in human drugs (Final revised guidance). U.S. Department of Health & Human Services. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs
- European Medicines Agency. (n.d.). Nitrosamine impurities: Scientific Q&A (Article 5(3) opinion). European Medicines Agency. https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/referral-procedures-human-medicines/nitrosamine-impurities/nitrosamine-impurities-guidance-marketing-authorisation-holders


