Introduction
Nitrosamine Testing in Chloroquine has become a critical requirement for pharmaceutical manufacturers across the world. Authorities like the U.S. FDA, Health Canada, and the European Medicines Agency (EMA) are closely monitoring the presence of these harmful impurities. For makers of Chloroquine active pharmaceutical ingredients (API) and finished medicines, it is essential to have reliable systems to detect, measure, and reduce nitrosamine levels, including Nitrosamine Drug Substance-Related Impurities (NDSRIs). Ignoring these risks can lead to costly product recalls and serious patient safety concerns. ResolveMass Laboratories Inc., with expertise in advanced nitrosamine impurity testing, supports end-to-end risk assessment and regulatory compliance.
Understanding the Synthesis of Chloroquine
Chloroquine (C₁₈H₂₆ClN₃) is typically created by reacting 4,7-dichloroquinoline with an aminoalkyl side chain such as 4-diethylamino-1-methylbutylamine. The process often uses solvents like dimethylformamide (DMF) or dimethylacetamide (DMA) along with certain catalysts or bases. These conditions can sometimes introduce amine-containing substances that may later form nitrosamines. The tertiary diethylamino group in Chloroquine’s structure makes it vulnerable to nitrosation if nitrosating agents are present. Even small amounts of impurities in raw materials can trigger this reaction. This is why manufacturers must monitor solvent quality, raw material purity, and reaction conditions closely to reduce the chances of nitrosamine formation.
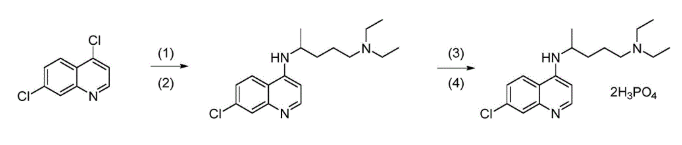
Zhangjiagang Weisheng Biological Medical Co., Ltd. (2021). Synthetic method of high-purity chloroquine phosphate (China Patent No. CN112300071A). China National Intellectual Property Administration. https://patents.google.com/patent/CN112300071A/en
Nitrosamine Risk Assessment in Chloroquine API Production
During the API manufacturing process, several steps can increase the chance of nitrosamine contamination:
- The intermediate 4-diethylamino-1-methylbutylamine can react with nitrites to form N-nitrosodiethylamine (NDEA), a known carcinogen.
- Using DMA or DMF in certain acidic or nitrosating conditions can lead to N-nitrosodimethylamine (NDMA).
- Other side reactions may form N-nitrosoethylbutylamine (NEBA) or N-nitrosodi-n-butylamine (NDBA).
- Piperidine-based byproducts can generate N-nitrosopiperidine (NPIP).
- Reusing solvents without proper purification may carry nitrosamines from previous batches.
Following Good Manufacturing Practices (GMP) is key to preventing these risks. This includes checking nitrite levels in materials, controlling solvent reuse, and carrying out in-process testing. Using real-time Nitrosamine Testing in Chloroquine production lines can help detect problems before they become serious.
Nitrosamine Risk in Chloroquine Finished Dosage Forms
Even after the API is produced, nitrosamine risks continue during tablet or capsule manufacturing:
- The diethylamino group in Chloroquine can react slowly with nitrites in certain excipients like starch or cellulose.
- Some excipients, such as lactose or povidone, can contain nitrites that react with the API during storage.
- Moisture and oxygen exposure can speed up nitrosamine formation.
- Combining nitrate-rich binders with alkaline lubricants can create higher NDMA or NDEA levels.
A thorough excipient risk review, regular supplier checks, and using protective packaging can reduce these risks. Nitrogen flushing and controlled storage conditions help keep nitrosamines from forming over time.
Known Nitrosamine Impurities in Chloroquine
Based on known chemical reactions and breakdown patterns, the following nitrosamines may be found in Chloroquine APIs or finished products, aligning with NDSRI regulatory listings from Health Canada, FDA, and EMA:
- N-Nitrosodiethylamine (NDEA)
- N-Nitrosodimethylamine (NDMA)
- N-Nitrosoethylbutylamine (NEBA)
- N-Nitrosodi-n-butylamine (NDBA)
- N-Nitrosopiperidine (NPIP)
- N-Nitrosodiisopropylamine (NDIPA)
High-sensitivity testing methods like LC-MS/MS and GC-MS can detect even very small amounts of these impurities. Routine Nitrosamine Testing in Chloroquine ensures compliance and strengthens consumer confidence. View our laboratory capabilities.
Why Compliance is Urgent
Regulatory agencies have made it clear (Health Canada limits) that nitrosamines like NDMA and NDEA cannot exceed strict safety limits. If these impurities are found above allowed levels, companies may face recalls, loss of licenses, and major financial loss. ResolveMass Laboratories provides comprehensive nitrosamine CRO support, from early-stage risk checks to validated test methods and long-term stability monitoring, helping manufacturers stay compliant and avoid costly setbacks.
Conclusion
Nitrosamine Testing in Chloroquine is not just a legal requirement — it is essential for patient safety and pharmaceutical quality. The chemical structure of Chloroquine makes it prone to nitrosation during both production and storage. By working with experienced labs like ResolveMass Laboratories, manufacturers can identify risks early, control nitrosamine levels, and deliver safe medicines to the market with confidence.
Contact ResolveMass Laboratories Today
Frequently Asked Questions (FAQs) on Nitrosamine Testing in Chloroquine
The biggest nitrosamine risk in Chloroquine API production comes from the tertiary diethylamino group in its chemical structure. When this reacts with nitrite contaminants, it can form N-nitrosodiethylamine (NDEA), a harmful compound. This reaction can happen during certain steps of synthesis if conditions are not well controlled.
Nitrosamine Testing in Chloroquine is essential because global health agencies set strict safety limits for these impurities. If nitrosamines like NDMA or NDEA are found above allowed levels, the product can be recalled, and the manufacturer may face penalties. Testing ensures the medicine meets quality standards before reaching patients.
Yes, reusing solvents without proper purification can raise nitrosamine risk. Contaminated solvents may carry over nitrosamines from previous batches or allow them to form again during processing. Careful cleaning and testing of recovered solvents are important for safety.
NDMA can appear in Chloroquine if certain solvents like DMF or DMA are used in conditions that promote nitrosation. While it may not be present in all batches, it is considered a possible impurity and is closely monitored due to its high toxicity.
The choice of raw materials, reaction steps, solvents, and storage conditions can all affect which NDSRIs form in Chloroquine. Even small changes in pH, temperature, or reagent purity can change the impurity profile. Consistent process control helps reduce these risks.
Yes, agencies like the FDA and EMA have issued guidance on nitrosamine risks in medicines, including Chloroquine. While specific findings may vary by manufacturer, the overall message is that testing and control measures are required to keep levels within safe limits.
Regular Nitrosamine Testing in Chloroquine can detect impurities early, before the medicine is released to the market. This allows manufacturers to fix problems and avoid selling unsafe products. By catching issues in time, companies can protect patients and prevent costly recalls.
References
- Zhangjiagang Weisheng Biological Medical Co., Ltd. (2021). Synthetic method of high-purity chloroquine phosphate (China Patent No. CN112300071A). China National Intellectual Property Administration. https://patents.google.com/patent/CN112300071A/en
- U.S. Food and Drug Administration. (2024, September). Control of nitrosamine impurities in human drugs (Final revised guidance). U.S. Department of Health & Human Services. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/control-nitrosamine-impurities-human-drugs
- Health Canada. (2025, August 1). Nitrosamine impurities in medications: Guidance. Government of Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities/medications-guidance.html


