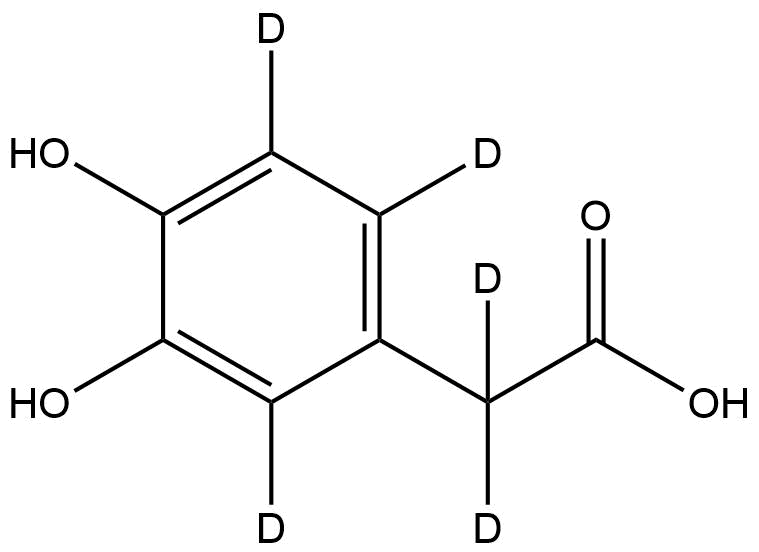
3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid | CAS No.: 60696-39-1 | Deuterated DOPAC
Chemical Name: 3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid
Common Name: DOPAC-d5
Molecular Formula: C8D5H3O4
Molecular Weight: 172.17 g/mol
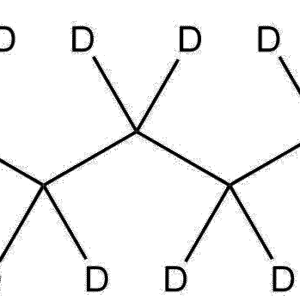
Isotopic Labeling: 5 deuterium atoms (3 on the aromatic ring, 2 on the side-chain methylene group)
Synonyms: Deuterated DOPAC, DOPAC-2,2,3,4,5-d5, 3,4-Dihydroxyphenylacetic acid-d5, Dopamine metabolite-d5
Isotopic Enrichment: ≥98 atom % D
Product Overview
3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid (CAS No. 60696-39-1) is a deuterium-labeled analog of 3,4-Dihydroxyphenylacetic acid (DOPAC), a primary oxidative metabolite of dopamine. This compound contains five deuterium atoms distributed over the aromatic and aliphatic regions, making it an ideal stable isotope-labeled internal standard for bioanalytical quantification of dopamine metabolites in biological samples such as plasma, cerebrospinal fluid, and brain tissue extracts.
As a critical biochemical marker in neurochemical and pharmacokinetic studies, DOPAC-d5 enables high-precision quantification of endogenous dopamine turnover and metabolism using LC-MS/MS and GC-MS analytical platforms. The isotopic labeling enhances its analytical specificity while retaining identical chromatographic and chemical properties as the non-deuterated DOPAC.
Structural and Chemical Features
3,4-Dihydroxyphenylacetic acid belongs to the catecholamine metabolite class, characterized by the presence of two hydroxyl groups at the 3- and 4-positions on the aromatic ring and an acetic acid moiety attached at the 1-position. In the deuterated derivative (3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid, three hydrogens on the aromatic ring and two on the α-methylene group are replaced with deuterium atoms, increasing the molecular weight by five daltons.
This isotopic substitution provides several analytical and physicochemical advantages:
-
Stable isotope signature allowing accurate quantitation via mass spectrometry
-
Minimal alteration of retention time compared to native DOPAC
-
Reduced isotopic exchange under physiological and analytical conditions
-
Distinct m/z (mass-to-charge) separation for unambiguous peak identification
The chemical stability of DOPAC-d5 under typical storage and analytical conditions ensures reliable use as a long-term analytical standard.
Applications
1. Neurochemical and Dopaminergic Research
3,4-Dihydroxyphenylacetic acid is a key dopamine metabolite, and its deuterated form (DOPAC-d5) is indispensable for monitoring dopamine catabolism in neurotransmitter turnover studies. Researchers employ this compound to investigate:
-
Dopaminergic neuron activity and degradation pathways
-
Enzymatic reactions catalyzed by monoamine oxidase (MAO)
-
Neurochemical imbalances in disorders such as Parkinson’s disease, schizophrenia, and depression
By using DOPAC-d5 as an internal standard, scientists can achieve highly accurate, reproducible quantification of endogenous DOPAC in complex biological matrices.
2. LC-MS/MS and GC-MS Quantification
In bioanalytical and pharmacokinetic studies, DOPAC-d5 is widely used as an internal standard in liquid chromatography-tandem mass spectrometry (LC-MS/MS) or gas chromatography-mass spectrometry (GC-MS) workflows. Its structural and retention-time similarity to DOPAC ensures equivalent ionization efficiency, while the 5 Da mass shift enables distinct detection.
This makes DOPAC-d5 essential for:
-
Quantitative neurotransmitter profiling
-
Pharmacometabolomics and biomarker discovery
-
Drug metabolism and toxicokinetic (DMTK) studies
3. Isotope Dilution Analysis
In isotope dilution assays, DOPAC-d5 is spiked into biological samples as a calibration reference to correct for matrix effects, sample loss, or instrumental variability. This technique provides unmatched accuracy and reproducibility, particularly in trace-level quantitation of catecholamine metabolites in blood, urine, or brain homogenates.
4. Pharmaceutical and Clinical Research
Pharmaceutical companies use 3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid in the validation of drug efficacy and neuropharmacological profiling of compounds targeting dopaminergic systems. Its precise quantitation allows the correlation of dopamine metabolism rates with therapeutic responses or drug-induced neurotoxicity.
Technical Specifications
Each batch of 3,4-Dihydroxyphenyl-d3)acetic-2,2-d2 Acid is validated using LC-MS/MS, ¹H/²H NMR, and FTIR spectroscopy to confirm isotopic labeling, purity, and identity.
Handling and Safety
Although DOPAC-d5 is non-radioactive and chemically stable, it should be handled with standard laboratory safety procedures. Avoid direct contact, inhalation, or ingestion. Use gloves and eye protection during handling and store in tightly sealed containers to prevent degradation. Refer to the Safety Data Sheet (SDS) for detailed hazard and precautionary information.
Packaging and Availability
-
Pack Sizes: 10 mg, 25 mg, 50 mg, 100 mg (custom sizes available upon request)
-
Lead Time: 2–4 weeks
-
Packaging: Supplied in amber glass vials under inert gas with tamper-proof sealing
Bulk and custom-synthesis options are available for research institutions and pharmaceutical companies requiring high isotopic purity or modified labeling patterns.
Why Choose ResolveMass Laboratories Inc.?
At ResolveMass Laboratories Inc., we specialize in the synthesis, purification, and analytical characterization of deuterated and isotopically labeled compounds for pharmaceutical, biochemical, and academic research. Our ISO 9001:2015-certified facility ensures precise isotopic enrichment, stringent analytical validation, and consistent lot-to-lot reproducibility.
We provide end-to-end support—from custom synthesis of labeled metabolites to analytical method development and validation assistance—to meet the demanding requirements of neurochemical and pharmacokinetic studies.
Read below articles on Deuterated Polymers:
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Reviews
There are no reviews yet.