Introduction:
Custom Organic Synthesis Process Development is a fundamental part of modern chemical research and development, supporting critical advancements in pharmaceuticals, materials science, and chemical engineering. Small-scale synthesis enables researchers to create and evaluate new compounds efficiently while ensuring reproducibility and precision. Developing reliable synthesis methods at this stage is essential for innovation and successful scale-up. This blog explores the key principles, tools, and best practices involved in Custom Organic Synthesis Process Development, highlighting its importance in advancing new technologies.
Role of Custom Organic Synthesis in Modern Research
Custom organic synthesis has become an essential service in modern scientific innovation, especially when commercially available compounds do not meet specific research requirements. Scientists often need molecules with unique structural features, uncommon functional groups, or precise stereochemistry. In such cases, tailored synthesis allows researchers to obtain compounds designed specifically for their experimental objectives. This level of customization enables accurate evaluation of chemical behavior, biological activity, and material performance.
In addition, custom synthesis supports faster research progress by allowing modifications to molecular structures based on experimental findings. Researchers can refine compounds to improve their effectiveness, stability, or selectivity. This flexibility is particularly important in drug development, where small structural changes can significantly influence therapeutic outcomes. As a result, custom organic synthesis plays a central role in advancing scientific discovery and developing next-generation products.
Explore more – Custom Synthesis
Introduction to Small Scale Organic Synthesis
Small-scale organic synthesis involves the preparation of organic compounds in quantities ranging from milligrams to grams. This scale is particularly relevant for research and development (R&D) settings where new compounds are synthesized for initial testing and analysis before scaling up for industrial production. The significance of small-scale synthesis extends to:
- Drug Discovery: Synthesizing potential pharmaceutical compounds for preliminary biological evaluation, enabling rapid identification of promising drug candidates.
- Materials Science: Creating novel materials for testing and characterization, facilitating the development of innovative materials with unique properties.
- Chemical Engineering: Conducting feasibility studies and optimizing reactions on a small scale to minimize resource expenditure before large-scale application.
Importance of Route Planning and Feasibility Evaluation
Careful route planning is a critical early step in small-scale synthesis process development. Chemists evaluate different synthetic pathways to determine which approach offers the best balance of efficiency, safety, and practicality. This involves considering the number of reaction steps, expected yields, availability of starting materials, and ease of purification. Selecting an efficient route helps reduce time, costs, and experimental complications during development.
Feasibility evaluation also allows researchers to identify potential challenges before beginning laboratory work. Some reactions may require harsh conditions, unstable intermediates, or difficult purification steps. By recognizing these challenges early, chemists can adjust their approach and select more practical alternatives. This proactive planning improves success rates and ensures smoother progression toward the desired compound.
Key Considerations in Process Development
Developing an effective small-scale organic synthesis process requires meticulous attention to several critical factors:
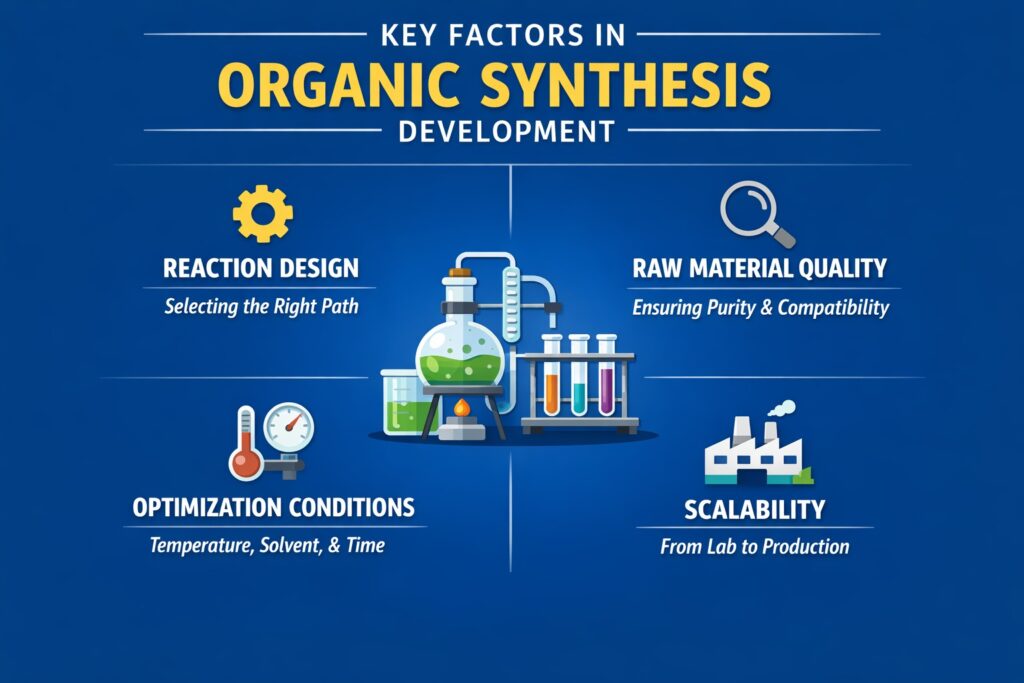
A. Reaction Design and Selection
Choosing the appropriate reaction is fundamental to the success of the synthesis process. Key considerations include:
- Reaction Type: Selecting the type of reaction, such as addition, substitution, elimination, or rearrangement, based on the desired transformation.
- Reagent Selection: Ensuring compatibility of reagents with the reaction conditions and their availability and cost.
- Catalysts: Utilizing suitable catalysts to enhance reaction rates and selectivity while minimizing by-products.
Evaluation of Raw Material Quality and Compatibility
The quality of raw materials used in synthesis has a significant impact on reaction success and reproducibility. Impurities present in starting materials can interfere with reaction mechanisms, reduce yields, or generate unwanted by-products. Therefore, it is essential to verify the purity and stability of reagents before use. Using high-quality materials ensures consistent reaction performance and reliable experimental outcomes.
Compatibility between raw materials and reaction conditions must also be carefully considered. Some compounds may degrade under certain temperatures, pressures, or solvent environments. Understanding these properties allows chemists to select appropriate reaction parameters and prevent material loss. Proper evaluation of raw materials ultimately improves process reliability and product quality.
B. Optimization of Reaction Conditions
Optimizing reaction conditions is essential for achieving the best possible yield and purity. Important parameters to consider are:
- Temperature and Pressure: Adjusting these parameters to accelerate reaction rates and improve selectivity while maintaining safety and practicality.
- Solvent Selection: Choosing a solvent that dissolves reactants efficiently and supports the reaction environment, considering factors such as polarity, boiling point, and toxicity.
- Reaction Time: Determining the optimal duration for the reaction to proceed to completion without compromising product stability or yield.
Role of Reaction Monitoring in Process Development
Monitoring reactions in real time provides valuable insight into how the synthesis is progressing. Techniques such as thin-layer chromatography (TLC), sampling, or analytical testing allow chemists to track the conversion of starting materials into products. This information helps determine whether the reaction is proceeding as expected or if adjustments are required. Early detection of incomplete reactions or side products allows timely corrective actions.
Effective monitoring also helps determine the exact point at which the reaction should be stopped. Allowing reactions to continue too long may lead to product degradation or impurity formation. By identifying optimal reaction endpoints, chemists can maximize yield and maintain product quality. This level of control is essential for developing reliable and efficient synthesis processes.
C. Scalability
Ensuring that the small-scale synthesis process can be scaled up effectively is crucial for its practical application. This involves:
- Reproducibility: Achieving consistent results across multiple small-scale batches to ensure reliability and predictability.
- Yield Optimization: Maximizing yield to make the process economically viable while minimizing waste and by-products.
- Purity: Ensuring the synthesized product meets stringent purity standards required for its intended application.
Techniques and Tools in Small Scale Synthesis
Modern small-scale synthesis leverages advanced techniques and tools to enhance efficiency, accuracy, and reproducibility:
A. Automated Synthesis Platforms
Automated synthesis platforms offer significant advantages in small-scale synthesis:
- Precision Control: Providing accurate control over reaction conditions, such as temperature, pressure, and reagent addition, to ensure consistency.
- High Throughput: Enabling the parallel synthesis of multiple compounds, accelerating the screening and optimization process.
- Data Collection: Facilitating real-time monitoring and data analysis, allowing for immediate adjustments and optimization.
Advantages of Miniaturization in Chemical Development
Miniaturization offers several advantages in small-scale organic synthesis, particularly during early research stages. Working with smaller quantities reduces the consumption of expensive reagents and minimizes waste generation. This allows researchers to conduct multiple experiments efficiently without excessive resource use. Miniaturization also makes it easier to test different reaction conditions and identify optimal parameters quickly.
Another benefit of miniaturization is improved safety during experimental work. Smaller reaction volumes reduce the risk associated with hazardous chemicals and exothermic reactions. This enables researchers to explore new chemical transformations with greater confidence. As a result, miniaturized synthesis supports faster innovation while maintaining safety and efficiency.
B. Analytical Techniques
Advanced analytical techniques are essential for characterizing reaction products and optimizing synthesis processes:
- Mass Spectrometry (MS): Identifying and quantifying reaction products with high sensitivity and specificity, providing critical information on molecular weight and structure.
- Nuclear Magnetic Resonance (NMR): Elucidating molecular structures and confirming the identity and purity of synthesized compounds.
- High-Performance Liquid Chromatography (HPLC): Separating and purifying compounds, enabling the isolation of pure products from reaction mixtures.
Case Study: Developing a Small-Scale Synthesis Process
Consider a case study where a new pharmaceutical compound is being developed. The process involves several steps:
Step 1: Reaction Selection
- Reaction Type: Selective hydrogenation is chosen to reduce a specific functional group without affecting other parts of the molecule.
- Reagents: Selection of a suitable hydrogen source (e.g., hydrogen gas or transfer hydrogenation reagent) and a compatible catalyst (e.g., palladium on carbon).
Step 2: Optimization
- Temperature: Conduct trials at various temperatures to identify the optimal condition that maximizes reaction rate and selectivity.
- Solvent: Screen different solvents (e.g., ethanol, methanol, toluene) to find the one that provides the best solubility for reactants and supports the desired reaction pathway.
- Pressure: Optimize hydrogen pressure to ensure efficient hydrogenation without over-reduction or side reactions.
Step 3: Analytical Verification
- Mass Spectrometry (MS): Use MS to verify the molecular weight and identify any by-products or impurities.
- Nuclear Magnetic Resonance (NMR): Employ NMR to confirm the structure and purity of the synthesized compound, ensuring the correct functional groups are present.
- High-Performance Liquid Chromatography (HPLC): Utilize HPLC to separate and quantify the product, ensuring high purity and yield.
Step 4: Scalability Testing
- Reproducibility: Produce multiple batches at the small scale to ensure consistent results, confirming the reliability of the optimized conditions.
- Yield Optimization: Adjust reaction parameters based on analytical data to maximize yield and minimize by-products, ensuring economic viability.
- Purity: Ensure the product meets the required purity standards for its intended pharmaceutical application, implementing additional purification steps if necessary.
Technology Integration in Modern Synthesis Laboratories
Modern synthesis laboratories increasingly rely on advanced technologies to improve efficiency and accuracy. Digital tools help researchers record, analyze, and manage experimental data more effectively. Automated equipment ensures precise control over reaction conditions, reducing human error and improving reproducibility. These technologies allow chemists to focus more on innovation and less on repetitive manual tasks.
Technology integration also enhances collaboration and knowledge sharing between research teams. Digital records make it easier to review past experiments, identify trends, and optimize processes. This improves overall productivity and accelerates development timelines. As technology continues to evolve, its role in synthesis process development will become even more important.
Conclusion
Small-scale organic synthesis process development is a complex yet vital part of modern chemistry. By carefully designing reactions, optimizing conditions, and leveraging advanced techniques, chemists can efficiently develop new compounds and processes. This approach not only accelerates innovation in various fields but also ensures that processes are scalable and economically viable.
At ResolveMass Laboratories, we specialize in custom synthesis and analytical services, leveraging our expertise to support the development of small-scale synthesis processes. Our state-of-the-art facilities and experienced team are dedicated to providing high-quality, reliable solutions tailored to meet the unique needs of our clients.
GET IN TOUCH
For more information on our services and how we can assist in your synthesis process development, please visit ResolveMass Laboratories and contact us.
FAQs – Custom Organic Synthesis Process Development
Custom organic synthesis is the process of designing and producing chemical compounds based on specific research or industrial requirements. It allows scientists to obtain molecules that are not commercially available or need unique modifications. This approach helps researchers achieve precise project goals efficiently.
Process development ensures that the synthesis method is efficient, reproducible, and scalable for future production. It helps optimize reaction conditions, improve yield, and reduce impurities. This stage is essential for transforming laboratory reactions into reliable manufacturing processes.
Purity is confirmed using analytical techniques such as NMR, HPLC, and mass spectrometry. These methods help identify the compound structure and detect impurities. Proper analysis ensures the synthesized material meets required quality standards.
Challenges include selecting the best synthetic route, controlling reaction conditions, and minimizing by-products. Some compounds may also involve unstable intermediates or difficult purification steps. Overcoming these challenges requires expertise and careful experimental design.
Custom synthesis enables researchers to produce new drug candidates for testing and evaluation. It ensures a reliable supply of molecules needed for biological studies. This accelerates the research process and supports faster development of new medicines.
Yes, processes developed on a small scale can be optimized for large-scale production. This involves improving reaction efficiency, ensuring safety, and maintaining consistent product quality. Successful scale-up makes commercial manufacturing possible.
The cost depends on factors such as synthesis complexity, number of steps, raw material availability, and purification requirements. Highly complex molecules require more time and resources. Scale and purity specifications also influence overall cost.
Reference
- American Chemical Society. (n.d.). Custom organic synthesis. Chemical & Engineering News. https://pubs.acs.org/doi/abs/10.1021/cen-09034-ad13
- Denmark, S. E. (2018). Organic synthesis: Wherefrom and whither? (Some very personal reflections). Israel Journal of Chemistry, 58(1–2), 61–72. https://pmc.ncbi.nlm.nih.gov/articles/PMC6010076/
- Ingle, R., & Waghchaure, R. (2025). The review article on organic synthesis. International Journal of Pharmaceutical Sciences, 3(12), 1095–1117. https://www.ijpsjournal.com/article/The+Review+Article+on+Organic+Synthesis
Regulatory Requirements for Peptide Sameness Study (FDA & Health Canada)
Introduction: FDA Peptide Sameness Study Requirements define the scientific and regulatory framework needed to demonstrate…
Nitrosamine Testing in Controlled Substances and Highly Potent APIs (HPAPIs)
Introduction: Why Nitrosamine Testing Highly Potent APIs Requires Specialized Expertise Nitrosamine Testing Highly Potent APIs…
Case Study: Reformulation Strategy to Eliminate Nitrosamine Risk Without Changing Bioequivalence
Introduction: Applying a Nitrosamine Reformulation Strategy Without Compromising Bioequivalence A structured Nitrosamine Reformulation Strategy can…
Peptide Sameness Study vs Biosimilar Comparability: What’s the Difference?
Introduction: Peptide Sameness vs Biosimilar Comparability is one of the most misunderstood concepts in modern…
Case Study: Root Cause Investigation of Unexpected NDMA Spike in Finished Product
Introduction In this NDMA Root Cause Investigation Case Study, we examine a real-world scenario involving…
Selecting the Right Nitrosamine Testing CRO: A Technical Due Diligence Checklist
Introduction Nitrosamine Testing CRO Selection requires detailed scientific evaluation and regulatory awareness. Global authorities continue…