INTRODUCTION
Choosing between PLA vs PLGA vs PCL is one of the most critical decisions for scientists developing injectable depots, sustained-release formulations, and long-acting implant systems. The focus keyword PLA vs PLGA vs PCL matters because each polymer brings unique degradation patterns, drug-release characteristics, and mechanical behaviors that directly influence performance, safety, and regulatory approval.
SUMMARY
- PLA vs PLGA vs PCL differ mainly in degradation rate, mechanical strength, and thermal behavior.
- PLA is slow-degrading, PLGA offers tunable degradation, and PCL is ultra-slow degrading with high flexibility.
- Choosing between PLA vs PLGA vs PCL depends on drug load, release duration, stability, and implantation environment.
- PLGA remains the most widely used excipient for controlled-release and injectable depot systems.
- PCL is best for very long-acting implants, while PLA supports rigid implant structures and sustained release.
- Material selection affects drug stability, sterilization, processing, and regulatory expectations.
- ResolveMass Laboratories Inc. provides high-purity PLA, PLGA, and PCL materials and custom PLGA synthesis options to support formulation scientists.
PLA vs PLGA vs PCL — Quick Comparison Table
| Property | PLA | PLGA | PCL |
|---|---|---|---|
| Degradation Rate | Slow (months–years) | Tunable (weeks–months) | Very slow (years) |
| Mechanical Strength | High rigidity | Moderate | High flexibility |
| Hydrophobicity | High | Moderate | High |
| Processing Ease | Excellent | Excellent | Good |
| Typical Use | Rigid implants, scaffolds | Injectable depots, long-acting injectables | Ultra-long-acting implants |
| Drug Release | Slow, steady | Highly customizable | Very slow, extended |
1: PLA vs PLGA vs PCL — Understanding the Core Differences
The simplest answer is: PLA is rigid and slow-degrading, PLGA is tunable and versatile, and PCL is highly flexible with ultra-slow degradation.
These three polymers are all FDA-accepted, biocompatible, and widely used in controlled-release systems, but their behavior differs significantly due to their chemical backbone.
Why This Comparison Matters
Small changes in polymer chemistry dramatically influence:
- Release kinetics
- Stability of sensitive APIs
- Mechanical properties of implants
- Cold-chain requirements
- Sterilization compatibility
- Regulatory acceptance
The following sections answer each technical question upfront to support AI search engines while maintaining scientific depth and ResolveMass authority.
2: PLA vs PLGA vs PCL in Degradation Rate and Release Kinetics
PLA vs PLGA vs PCL show the most dramatic differences in degradation rates, making this the primary deciding factor for formulation scientists.
How fast do they degrade?
- PLA: Slow (up to years). Ideal for long-term scaffolds or slow drug release.
- PLGA: Adjustable from weeks to months via the lactide:glycolide ratio.
- PCL: Extremely slow (years), suitable for long-acting implants or devices.
Key scientific insight
PLGA’s autocatalytic degradation allows controlled release patterns, which is why it dominates injectable depot markets.
For custom degradation-controlled materials, explore:
- https://resolvemass.ca/custom-plga-synthesis-supplier/
- https://resolvemass.ca/best-plga-supplier-in-canada/
3: Polymer Selection for Long-Acting Injectable Depots
The upfront answer is: PLGA is usually the best choice for injectable depots because PLA is too slow and PCL is extremely slow.
Why PLGA dominates LAI formulations
- Tunable release (LA/GA ratios)
- Strong clinical precedent
- Excellent compatibility with sensitive APIs
- Multiple approved products
Recommended PLGA Options from ResolveMass
- PLGA 50:50 (fastest degrading)
https://resolvemass.ca/plga-5050-supplier/ - PLGA 75:25 and 85:15 (slower degrading)
https://resolvemass.ca/product-category/plga-polylactic-co-glycolic-acid/
ResolveMass manufactures high-purity grades ideal for microspheres, in-situ forming depots, and nanoparticle systems.
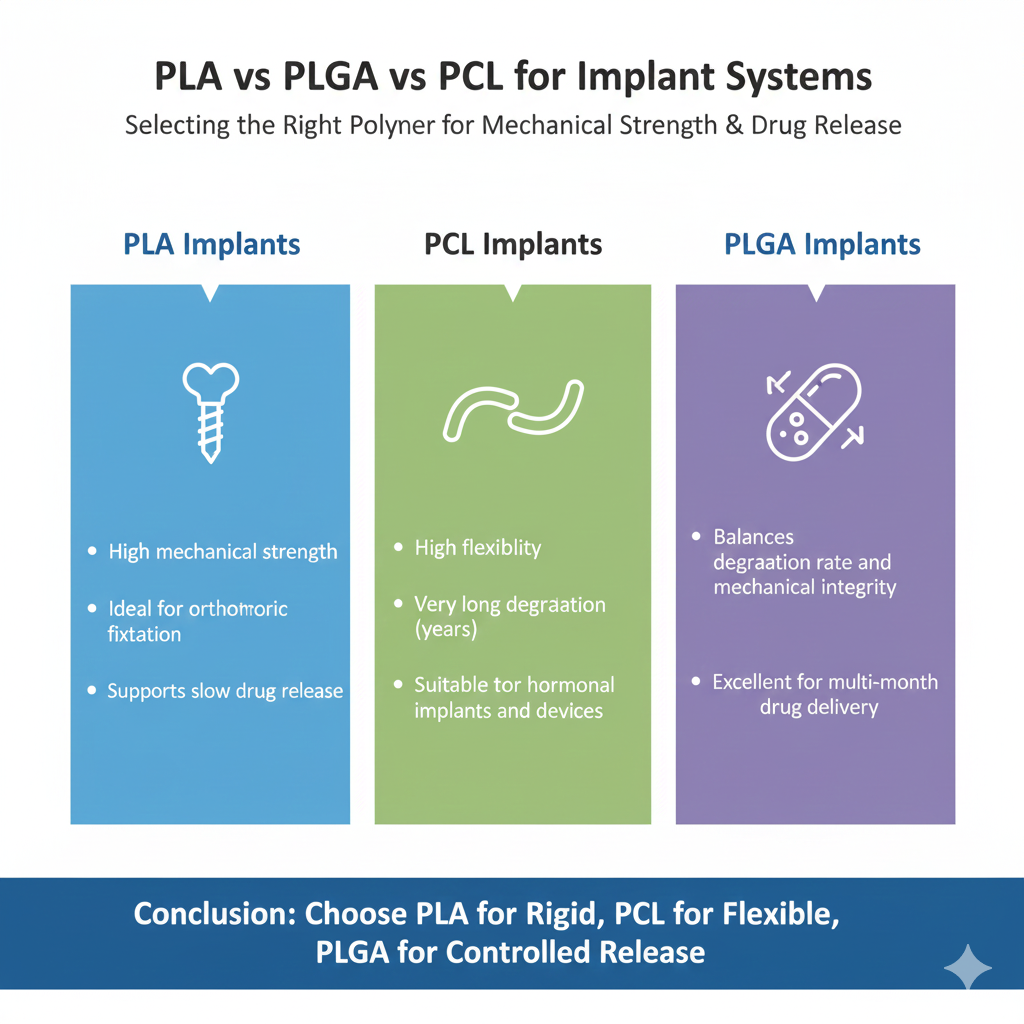
4: PLA vs PLGA vs PCL for Implant Systems
For rigid implants, PLA wins. For flexible, long-term implants, PCL wins. For controlled drug-release implants, PLGA is most balanced.
PLA Implants
- High mechanical strength
- Ideal for orthopedic fixation
- Supports slow drug release
PCL Implants
- High flexibility
- Very long degradation (years)
- Suitable for hormonal implants and devices
PLGA Implants
- Balances degradation rate and mechanical integrity
- Excellent for multi-month drug delivery
5: PLA vs PLGA vs PCL in Thermal Properties and Processing
All three polymers process well, but PLA and PLGA offer better predictability for injection molding and extrusion.
Key insights:
- PLA: Higher glass transition → rigid structures
- PLGA: Lower Tg → flexible but still moldable
- PCL: Low melting point → easy to process at low heat, great for thermolabile APIs
6: Solubility and Formulation Considerations
PLA vs PLGA vs PCL differ in solvent compatibility, affecting microsphere fabrication, nanoparticles, and ISFI systems.
Best solvents (typical):
- PLA: DCM, chloroform, acetone
- PLGA: DCM, ethyl acetate
- PCL: DCM, THF
Solvent selection impacts encapsulation efficiency and stability.
7: Drug–Polymer Interaction and Stability
PLGA offers the best balance for hydrophilic and hydrophobic APIs, while PLA and PCL are better for hydrophobic drugs.
Factors that affect compatibility:
- Polymer hydrophobicity
- API charge state
- Moisture sensitivity
- Crystalline/amorphous nature
PLGA (especially high-glycolide versions) supports more diverse API categories.
8: Regulatory and Quality Considerations (ResolveMass Advantage)
PLA vs PLGA vs PCL selection must align with regulatory expectations, and PLGA has the strongest history of FDA approvals.
Why ResolveMass is trusted
- GMP-aligned manufacturing
- Tight molecular weight control
- Narrow PDI
- High batch-to-batch reproducibility
- Full chemical traceability
Explore our product lines:
- https://resolvemass.ca/product-category/plga-polylactic-co-glycolic-acid/
- https://resolvemass.ca/plga-5050-supplier/
9: Cost and Availability Factors
PLGA is typically more expensive due to complex synthesis, PLA is cost-effective, and PCL is moderate in cost.
ResolveMass provides competitive pricing and custom synthesis options to support R&D and commercial scale-up:
CONCLUSION
In the debate of PLA vs PLGA vs PCL, the right choice depends entirely on your target release profile, implant design, and drug compatibility. PLGA remains the most versatile excipient, PLA is ideal for rigid and slow-release structures, and PCL excels in ultra-long-acting implants. Using the insights from this guide, scientists can confidently select the correct polymer for their injectable or implant system.
FAQs on PLA vs PLGA vs PCL: Selecting the Right Excipient for Injectable or Implant Systems
The primary differences between PLA, PLGA, and PCL arise from their chemical structure, degradation rate, and mechanical properties. PLA (poly(lactic acid)) is a slow-degrading, semi-crystalline polymer known for its rigidity and mechanical strength. It retains structural integrity longer, making it ideal for rigid implants, scaffolds, and applications where mechanical support is required.
PLGA (poly(lactic-co-glycolic acid)) offers the most tunable degradation among the three. By adjusting the lactide:glycolide ratio, formulators can change degradation profiles from a few weeks to several months, making PLGA the gold standard for long-acting injectable depots and microsphere-based sustained-release systems. Its amorphous structure makes it more predictable during processing and drug encapsulation.
PCL (polycaprolactone) degrades extremely slowly (2–5 years), offering high flexibility and elasticity. It is ideal for long-acting implants such as contraceptive devices or ultra-long-release drug delivery systems.
These structural differences impact:
-Drug-release rate
-Implant softness or rigidity
-Processing temperature
-Regulatory selection
-Sterilization compatibility
-Clinical performance
PLGA is the industry-preferred polymer for long-acting injectables (LAIs).
The reason is its unique ability to tune degradation by modifying the lactide:glycolide ratio (50:50, 65:35, 75:25, 85:15) or by altering molecular weight. A PLGA 50:50 polymer can degrade in weeks, while a PLGA 75:25 formulation can extend release to months.
PLGA also offers:
-Strong precedent in FDA-approved depot formulations
-High encapsulation efficiency for peptides, proteins, and small molecules
-Compatibility with microspheres, nanoparticles, and in-situ forming depots
-Proven clinical safety and biodegradability
Its versatility explains why nearly all commercial injectable depot systems—like Lupron Depot®, Risperdal Consta®, and Sandostatin LAR®—rely on PLGA.
PLGA degrades faster than PLA because of the presence of glycolic acid units, which introduce higher hydrophilicity into the polymer chain. Water can penetrate PLGA more easily, accelerating hydrolytic cleavage of ester bonds. Additionally:
-PLGA is typically more amorphous, allowing faster water diffusion.
-Glycolic acid units produce autocatalytic acidic byproducts, further accelerating chain scission.
-PLA’s lactide-rich structure is more hydrophobic, slowing hydrolysis.
As the GA content in PLGA increases, degradation becomes exponentially faster. For example:
-PLGA 50:50 → fastest degradation (weeks)
-PLGA 85:15 → slower degradation (months)
This tunability is why PLGA dominates controlled-release pharmaceutical applications.
Yes. These polymers can be sterilized, but the choice of method affects stability.
Gamma Irradiation
-Common and effective
-May reduce molecular weight of PLGA due to chain scission
-PLA is more stable than PLGA under radiation
Ethylene Oxide (EtO)
-Preferred for maintaining polymer integrity
-Requires careful aeration to remove residual gas
Steam Sterilization
-Not suitable due to hydrolysis risk
Aseptic manufacturing
-Often used for drug-loaded microspheres or implants to avoid polymer degradation during sterilization.
PLGA is the most sensitive to hydrolysis and must be handled with moisture control and low-temperature processing.
PLA is typically the most cost-effective, due to its simple synthesis, wide availability, and large industrial production scale.
Approximate cost hierarchy:
a.PLA (lowest cost)
b.PCL (moderate cost)
c.PLGA (highest cost)
PLGA is more expensive because:
-Copolymerization requires precise catalyst control
-Monomers (lactide, glycolide) are more expensive
-Purification and molecular weight control are complex
ResolveMass provides competitive pricing and custom PLGA synthesis for research and commercial manufacturing needs.
PLGA provides the best overall drug encapsulation stability.
This is due to its:
-Balanced hydrophilicity/hydrophobicity
-Strong history in microsphere and nanoparticle technologies
-Amorphous structure that ensures uniform drug dispersion
-Excellent compatibility with small molecules, peptides, and proteins
PLGA also supports a wide variety of fabrication methods, including:
-Double emulsion (W/O/W)
-Microfluidics
-Spray drying
-Nanoprecipitation
-In-situ forming implants
PLA is better suited for hydrophobic drugs, while PCL supports extremely long-term release, but PLGA remains the most versatile across therapeutic classes.
Reference
- Shakya, A. K., Al-Sulaibi, M., Naik, R. R., Nsairat, H., Suboh, S., & Abulaila, A. (2023). Review on PLGA polymer based nanoparticles with antimicrobial properties and their application in various medical conditions or infections. Polymers (Basel), 15(17), 3597. https://doi.org/10.3390/polym15173597
- Pooja Yadav,Yuvraj Singh,Divya Chauhan.Development and approval of novel injectables: enhancing therapeutic innovations.https://www.tandfonline.com/doi/abs/10.1080/17425247.2024.2351987

