Introduction
When your research, product development, or regulatory compliance depends on reliable analytical data, selecting the right GC-MS analysis services becomes mission-critical. Gas Chromatography-Mass Spectrometry (GC-MS) remains the gold standard for identifying and quantifying volatile and semi-volatile compounds across pharmaceutical, environmental, food safety, and chemical industries. However, not all GC-MS analysis services deliver the same level of accuracy, reliability, or speed that your project demands.
This comprehensive guide walks you through the essential criteria for evaluating and comparing GC-MS analysis services across North America, ensuring you partner with a laboratory that meets your specific analytical requirements while delivering results you can trust.
Summary
Finding the best GC-MS analysis services in North America requires evaluating multiple critical factors beyond just price. This comprehensive guide helps you compare laboratories based on accuracy, turnaround time, accreditation, and technical capabilities.
Key Takeaways:
- Accuracy Standards: Top GC-MS analysis services maintain ISO/IEC 17025 accreditation and achieve detection limits below 1 ppb
- Turnaround Time: Industry-leading labs deliver standard results in 3-5 business days, with rush services available in 24-48 hours
- Quality Indicators: Look for labs with method validation documentation, proficiency testing participation, and transparent quality control protocols
- Technology Requirements: Modern GC-MS analysis services should utilize triple quadrupole or high-resolution mass spectrometry systems
- Cost vs. Value: The cheapest option rarely provides the accuracy and reliability needed for critical applications
- Communication: Leading laboratories provide dedicated project managers and technical consultation throughout the analysis process
Looking for reliable GC-MS analysis services?
Contact ResolveMass Laboratories for expert testing, fast turnaround, and highly accurate results.
https://resolvemass.ca/gcms-analysis-service/
1: Understanding GC-MS Analysis Services: What Sets Leading Labs Apart
Leading GC-MS analysis services distinguish themselves through superior instrumentation, validated methodologies, and proven quality systems. The difference between adequate and exceptional analytical services often determines project success or failure.
https://resolvemass.ca/working-principle-of-gc-ms/
Core Components of Quality GC-MS Analysis Services
Superior GC-MS analysis services incorporate several fundamental elements:
Instrumentation Quality:
- Triple quadrupole MS/MS systems for enhanced selectivity
- High-resolution accurate mass spectrometry capabilities
- Regular calibration and maintenance protocols
- Multiple instrument platforms for method redundancy
Method Development Expertise:
- Custom method development for unique matrices
- Method validation following ICH, FDA, or EPA guidelines
- Matrix-matched calibration approaches
- Proven extraction and sample preparation techniques
Quality Assurance Programs:
- Comprehensive standard operating procedures
- Regular proficiency testing participation
- Robust chain of custody documentation
- Multi-level review processes for data integrity
Explore real-world applications of GC-MS across regulated and research environments:
https://resolvemass.ca/applications-of-gcms/
2: Key Factors for Comparing GC-MS Analysis Services
1. Accuracy and Precision: The Foundation of Reliable Data
The accuracy of GC-MS analysis services should be verified through documented method validation studies demonstrating precision, accuracy, linearity, and detection limits appropriate for your application. Request validation reports before committing to a service provider.
https://resolvemass.ca/gcms-method-development-service/
Metrics to Evaluate:
| Quality Metric | Industry Standard | What to Ask For |
|---|---|---|
| Precision (RSD) | <15% for most applications | Intra-day and inter-day precision data |
| Accuracy (Recovery) | 80-120% for trace analysis | Recovery studies in relevant matrices |
| Detection Limits | Matrix and analyte dependent | LOD and LOQ documentation |
| Linearity (R²) | >0.995 minimum | Calibration curve data |
Questions to Ask Potential Labs:
- What is your laboratory’s measurement uncertainty for target analytes?
- How frequently do you participate in proficiency testing programs?
- Can you provide examples of method validation reports?
- What are your typical recovery rates for compounds similar to mine?
- How do you handle out-of-specification results?
2. Turnaround Time: Balancing Speed with Quality
Most reputable GC-MS analysis services offer standard turnaround times of 5-10 business days, with rush services available in 24-72 hours for urgent projects. However, the fastest turnaround means nothing if accuracy is compromised.
Turnaround Time Benchmarks:
Standard Service (5-10 business days):
- Routine environmental screening
- Food safety compliance testing
- Research and development applications
- Non-time-sensitive quality control
Expedited Service (3-5 business days):
- Product release testing
- Investigation of quality events
- Regulatory submission support
- Time-sensitive research milestones
Rush Service (24-48 hours):
- Emergency contamination investigations
- Critical manufacturing decisions
- Regulatory inspections
- Crisis management situations
Important Considerations:
- Does rush service maintain the same quality standards?
- Are rush fees reasonable for your budget?
- What is the lab’s on-time delivery rate?
- How does the lab communicate delays proactively?
3. Accreditation and Compliance: Ensuring Regulatory Acceptance
Top-tier GC-MS analysis services maintain ISO/IEC 17025 accreditation from recognized accreditation bodies, ensuring their results meet international quality standards. This accreditation is often mandatory for regulatory submissions.
Essential Accreditations and Certifications:
- ISO/IEC 17025: International standard for testing and calibration laboratories
- GLP Compliance: Required for non-clinical safety studies
- FDA Registration: For laboratories supporting pharmaceutical applications
- CLIA Certification: For clinical diagnostic testing
- EPA Certification: For environmental testing programs
- AOAC Accreditation: For food and agricultural testing
Red Flags to Watch For:
- Vague references to “quality systems” without specific accreditations
- Reluctance to share accreditation certificates
- Accreditations from unrecognized bodies
- Expired or suspended certifications
4. Technical Expertise and Method Capabilities
The best GC-MS analysis services employ PhD-level scientists with specialized expertise in your industry and application area. Technical knowledge directly impacts method selection, troubleshooting, and data interpretation.
Evaluating Laboratory Expertise:
Staff Qualifications:
- Educational background of technical staff
- Years of experience in relevant applications
- Publication records and conference presentations
- Industry-specific training and certifications
Method Library:
- Availability of standardized methods (EPA, AOAC, USP)
- Custom method development capabilities
- Experience with challenging matrices
- Validated methods for your specific analytes
Analytical Scope:
- Single or multi-residue analysis capabilities
- Quantitative and qualitative analysis
- Unknown identification services
- Impurity profiling and characterization
5. Instrumentation and Technology
Modern GC-MS analysis services should utilize current-generation mass spectrometry technology updated within the past 5-7 years. Older instruments may lack the sensitivity and resolution needed for trace-level detection.
Technology Assessment Checklist:
Mass Spectrometry Platforms:
- Single quadrupole MS (for routine screening)
- Triple quadrupole MS/MS (for complex matrices)
- High-resolution accurate mass (HRAM) systems
- GC-MS/MS with electron impact or chemical ionization
Supporting Instrumentation:
- Automated sample preparation systems
- Headspace and purge-and-trap samplers
- Thermal desorption units
- Multiple GC-MS systems for capacity and redundancy
Data Systems:
- Modern chromatography data systems (CDS)
- 21 CFR Part 11 compliance for electronic records
- Advanced data processing software
- Secure data storage and backup systems
3: Industry-Specific Considerations for GC-MS Analysis Services
Environmental Testing
Environmental GC-MS analysis services require EPA method certification and experience with complex environmental matrices. Look for laboratories with NELAP accreditation and experience in your specific environmental application.
methods. Pesticide residue analysis is a common application.
https://resolvemass.ca/pesticide-testing-services-using-gc-ms-in-canada/
Critical Capabilities:
- EPA Method 8260 (volatile organic compounds)
- EPA Method 8270 (semivolatile organic compounds)
- Low detection limits for groundwater and drinking water
- Experience with soil, sediment, and waste matrices
Pharmaceutical and Biopharmaceutical
Pharmaceutical GC-MS analysis services must operate under GMP/GLP conditions with validated methods suitable for regulatory submissions. FDA inspection readiness is essential.
https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/
Essential Requirements:
- Method validation per ICH guidelines
- Residual solvent analysis (ICH Q3C)
- Genotoxic impurity testing
- Extractables and leachables studies
- Method transfer capabilities
Food and Agricultural
Food safety GC-MS analysis services need AOAC accreditation and experience with diverse food matrices. Pesticide residue analysis and contamination screening are common applications.
Key Capabilities:
- Multi-residue pesticide analysis
- Mycotoxin screening
- Veterinary drug residues
- Flavor and fragrance profiling
- Contaminant identification
GC-MS analysis of plant extracts:
https://resolvemass.ca/gcms-analysis-of-plant-extract/
Complementary LC-MS analysis:
https://resolvemass.ca/lcms-analysis-of-plant-extract/
4: Cost Considerations: Understanding GC-MS Analysis Service Pricing
GC-MS analysis services typically range from $150-$500 per sample for routine analysis, with specialized testing reaching $1,000+ per sample. Multiple factors influence pricing beyond just the analytical technique.
Pricing Factors:
Sample Complexity:
- Simple matrices (solvents, standards) = lower cost
- Complex matrices (foods, biologicals) = higher cost
- Number of target analytes
- Required detection limits
Method Requirements:
- Standardized methods = lower cost
- Custom method development = higher cost
- Extensive validation requirements
- Special sample preparation needs
Service Level:
- Standard turnaround = standard pricing
- Rush service = 50-100% premium
- After-hours or weekend analysis
- Dedicated project support
Volume Considerations:
- Per-sample pricing decreases with volume
- Long-term agreements may offer discounts
- Retainer arrangements for regular testing
- Bundle pricing for method development plus testing
5: How to Evaluate Laboratory Communication and Customer Service
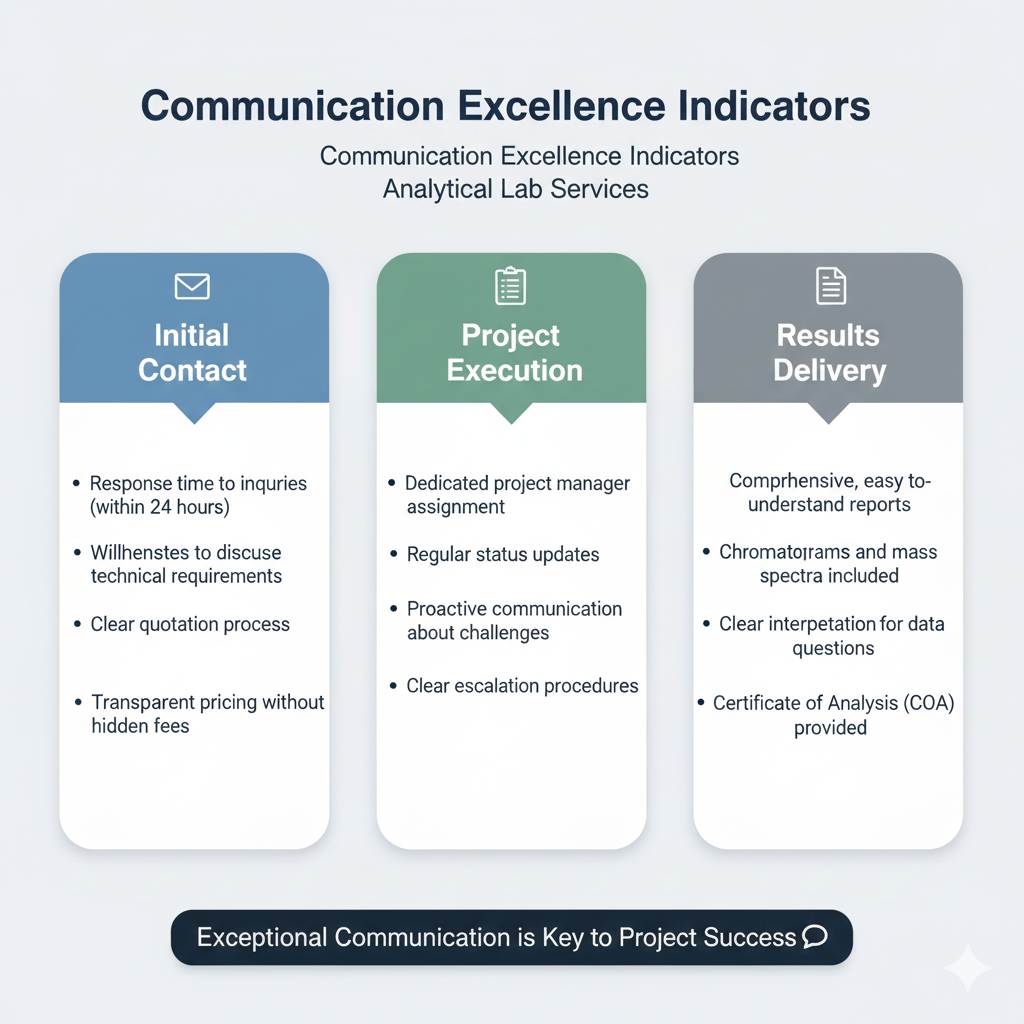
Responsive communication and transparent project management separate excellent GC-MS analysis services from mediocre ones. Technical expertise means little if you cannot access it when needed.
Communication Excellence Indicators:
Initial Contact:
- Response time to inquiries (within 24 hours)
- Willingness to discuss technical requirements
- Clear quotation process
- Transparent pricing without hidden fees
Project Execution:
- Dedicated project manager assignment
- Regular status updates
- Proactive communication about challenges
- Clear escalation procedures
Results Delivery:
- Comprehensive, easy-to-understand reports
- Chromatograms and mass spectra included
- Clear interpretation of results
- Technical support for data questions
- Certificate of Analysis (CoA) provided
- GC-MS analysis in Montreal
https://resolvemass.ca/gcms-analysis-in-montreal/ - Why ResolveMass is a top choice in Montreal, Canada
https://resolvemass.ca/gcms-analysis-in-montreal-canada-why-resolvemass-laboratories-inc-is-your-best-choice/ - GC-MS analysis services in the United States
https://resolvemass.ca/gcms-analysis-in-united-states/
6: Making Your Selection: A Step-by-Step Approach
Step 1: Define Your Requirements
Create a detailed specification document including:
- Target analytes and matrices
- Required detection limits
- Turnaround time needs
- Regulatory requirements
- Budget constraints
Step 2: Research Potential Labs
Identify GC-MS analysis services that specialize in your application area:
- Industry associations and directories
- Peer recommendations
- Published case studies
- Online reviews and testimonials
Step 3: Request Technical Capabilities
Ask for detailed information about:
- Accreditation scope
- Method validation documentation
- Instrumentation specifications
- Staff qualifications
- Quality control procedures
Step 4: Compare Proposals
Evaluate quotes based on:
| Evaluation Criteria | Weight | Notes |
|---|---|---|
| Technical capability | 35% | Can they meet your specifications? |
| Quality systems | 25% | Accreditations and certifications |
| Turnaround time | 20% | Realistic timelines with contingency |
| Cost | 15% | Total project cost, not just per-sample |
| Communication | 5% | Responsiveness and clarity |
Step 5: Request References
Contact previous clients about:
- Quality of results
- Timeliness of delivery
- Problem resolution
- Technical support
- Overall satisfaction
Step 6: Start with a Trial Project
Before committing to a large program:
- Submit test samples with known concentrations
- Evaluate report quality and turnaround
- Assess communication throughout the process
- Compare results against your specifications

7: Red Flags: Warning Signs of Substandard GC-MS Analysis Services
Be cautious of laboratories that:
Quality Concerns:
- Cannot provide accreditation documentation
- Lack documented method validation
- Show reluctance to discuss quality control
- Have no proficiency testing records
- Cannot explain their QC failure rates
Explore available service options here:
https://resolvemass.ca/gcms-analysis-service-2/
Communication Issues:
- Slow response times to inquiries
- Vague or evasive answers to technical questions
- No dedicated contact person
- Poor documentation practices
- Difficulty reaching technical staff
Operational Problems:
- Unrealistic turnaround promises
- Significantly below-market pricing
- Frequent missed deadlines
- High staff turnover
- Limited instrumentation capacity
8: The ResolveMass Laboratories Advantage
At ResolveMass Laboratories Inc., we have built our reputation on delivering GC-MS analysis services that exceed client expectations across accuracy, turnaround time, and technical support. Our North American facilities combine cutting-edge instrumentation with decades of collective expertise in gas chromatography-mass spectrometry applications.
Learn more about GC-MS technology:
https://resolvemass.ca/gas-chromatography-mass-spectrometry/
Why Leading Organizations Choose Our GC-MS Analysis Services:
Uncompromising Accuracy:
- ISO/IEC 17025 accredited testing
- Method validation per international standards
- Regular proficiency testing participation
- Multi-level data review processes
- Documented measurement uncertainty
Industry-Leading Turnaround:
- Standard service: 3-5 business days
- Rush service: 24-48 hours available
- 98%+ on-time delivery rate
- Real-time project status updates
- Flexible scheduling for urgent needs
Technical Excellence:
- PhD-level scientific staff
- Specialized expertise across industries
- Custom method development
- Advanced instrumentation fleet
- Comprehensive analytical capabilities
Client-Focused Service:
- Dedicated project managers
- Transparent communication
- Competitive pricing structures
- Flexible contract arrangements
- Technical consultation included
Conclusion
Selecting the right GC-MS analysis services partner requires careful evaluation of multiple factors beyond simple cost comparison. Accuracy, turnaround time, accreditation, technical expertise, and communication quality all contribute to successful analytical projects. By systematically assessing potential laboratories against the criteria outlined in this guide, you can identify GC-MS analysis services that deliver reliable, defensible results when you need them.
The investment in quality GC-MS analysis services pays dividends through reduced project risk, regulatory confidence, and faster time to market. Whether you need routine screening or complex method development, the right laboratory partnership becomes a competitive advantage for your organization.
Ready to experience the difference that expert GC-MS analysis services can make for your projects? ResolveMass Laboratories Inc. stands ready to support your analytical needs with the accuracy, speed, and technical excellence your work demands.
FAQs on GC-MS Analysis Services:
A top-tier GC-MS lab ensures accurate quantification, validated methods, strong quality controls, certified instrumentation, and reliable reproducibility. Accreditation (ISO/IEC 17025), expert analysts, and transparent reporting also indicate a reputable service.
Compare their method validation data, calibration procedures, detection limits (LOD/LOQ), reference standards used, and participation in proficiency testing programs. Ask for sample reports to evaluate clarity and consistency.
Modern instruments such as GC-MS/MS or high-resolution mass spectrometers provide superior sensitivity, lower noise, faster acquisition, and better compound identification—critical when analyzing trace-level compounds.
Turnaround time affects project timelines, regulatory submissions, and R&D decision-making. Leading labs offer expedited services (24–72 hours), while standard timelines range from 5–15 business days depending on complexity.
Look for ISO/IEC 17025 accreditation, GLP compliance (if applicable), and specific regulatory standards relevant to your industry (EPA, FDA, Health Canada, etc.). These ensure validated processes and defensible results.
Pricing varies due to instrumentation type, method development needs, sample complexity, data interpretation level, and turnaround time. Lower cost may indicate limited validation or slower processing.
Reference
- Prajita Pandey.LCMS vs. GCMS: When to Choose Each for Optimal Results in Your Analytical Chemistry.Jul 11, 2025.https://emerypharma.com/blog/lcms-vs-gcms-when-to-choose-each-for-optimal-results-in-your-analytical-chemistry/
- Diane Turner.GC-MS Principle, Instrument and Analyses and GC-MS/MS.February 16, 2024.https://www.technologynetworks.com/analysis/articles/gc-ms-principle-instrument-and-analyses-and-gc-msms-362513

