Introduction
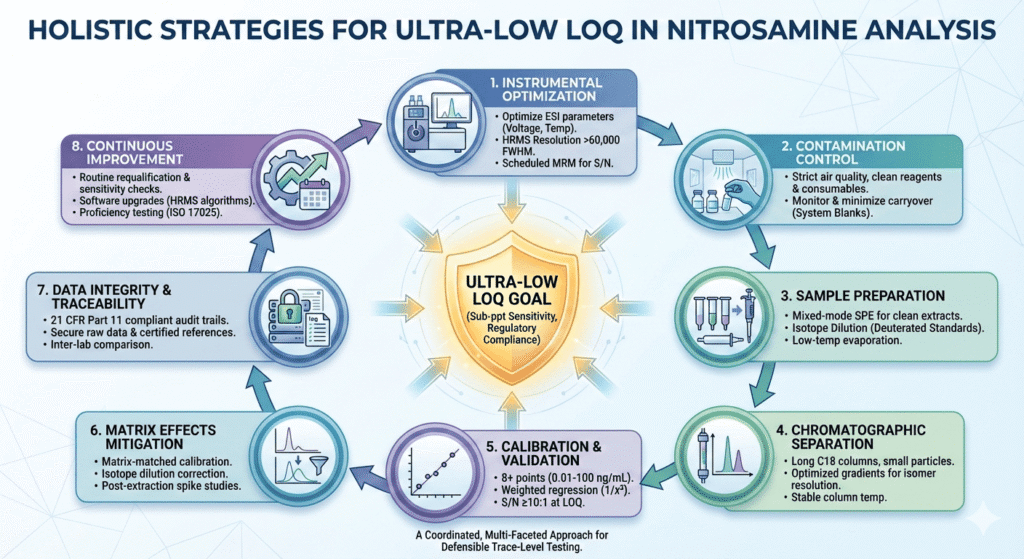
Achieving Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing requires a well-coordinated combination of analytical precision, instrument optimization, and contamination prevention. Nitrosamines are difficult to measure because they are widely present in the environment and regulated at extremely low levels. Laboratories must therefore manage sensitivity, selectivity, and reproducibility together, rather than in isolation.
Advanced chromatographic methods paired with high-resolution mass spectrometry (HRMS) allow laboratories to clearly distinguish nitrosamines from background interferences. At the same time, environmental controls such as air quality, solvent purity, and clean consumables are equally important. Without these controls, even the most advanced instruments may struggle to maintain ultra-low LOQs.
👉 To understand the broader analytical challenges, explore this overview of nitrosamine impurities in pharmaceuticals.
This article explains practical and validated strategies that help laboratories achieve and maintain ultra-low LOQs beyond regulatory limits. Each section focuses on a specific part of the analytical process, offering realistic insights based on laboratory best practices. Together, these strategies support consistent, compliant, and defensible nitrosamine testing.
👉 For a comprehensive service perspective, learn more about nitrosamine analysis solutions.
Summary
- Integration of automated sample prep and hybrid HRMS systems leads to unprecedented LOQ performance.
- Achieving ultra-low LOQ in nitrosamine testing demands precise control over sample preparation, instrument sensitivity, and contamination control.
- Key strategies include advanced chromatographic separation, high-resolution MS calibration, cleanroom-level sample handling, and matrix suppression mitigation.
- Method validation, calibration curve linearity, and traceability are essential for regulatory compliance.
- Data integrity and continuous method optimization ensure trust and reproducibility.
1. Instrumental Optimization for Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing
Instrument performance is the foundation of achieving ultra-low LOQs, especially when using LC-MS/MS or HRMS platforms. Sensitivity, resolution, and long-term stability directly determine how low a concentration can be reliably quantified. Proper system configuration ensures that trace-level signals remain clearly distinguishable from background noise.
Ion source optimization is particularly important. Adjusting electrospray ionization (ESI) parameters such as spray voltage, gas flow, and drying temperature improves ionization efficiency and minimizes signal suppression. Stable ionization conditions also enhance reproducibility across long analytical runs and multiple batches.
High-resolution mass spectrometers operating above 60,000 FWHM at m/z 200 allow clear separation of nitrosamine isomers and related impurities. Expanding the dynamic range through careful calibration ensures accurate quantitation at sub-ppt levels. Scheduled MRM (sMRM) further improves signal-to-noise ratios by reducing dwell-time competition.
👉 Discover how advanced detection platforms enhance sensitivity in LC-MS/MS nitrosamine testing.
Example Table: Optimization Parameters for Sub-ppt LOQ
| Parameter | Recommended Range | Impact on LOQ |
|---|---|---|
| ESI Source Voltage | 3500–4000 V | Improves ionization efficiency |
| Drying Gas Temp | 300–350°C | Prevents droplet clustering |
| Resolution (HRMS) | >60,000 FWHM | Separates nitrosamine isomers |
| Scan Cycle Time | <0.3 sec | Enhances peak definition |
2. Contamination Control and Background Reduction for Ultra-Low LOQ
Achieving Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing is not possible without strict contamination control. Nitrosamines can be introduced from many unexpected sources, making background management an ongoing responsibility. Even very low environmental exposure can increase baseline noise and compromise sensitivity.
Common contamination sources include laboratory air containing nitrogen oxides (NOx), impurities in solvents, plasticware additives, and residues in glassware. Sample carryover between injections can also falsely elevate results. Identifying and monitoring these risks is essential for reliable trace-level analysis.
Preventive measures include using low-nitrite solvents, certified nitrosamine-free consumables, and dedicated clean areas for sample preparation. Running system blanks after every few injections confirms that carryover is under control. Inert tubing, pre-column filters, and long-term background tracking help maintain a stable and defensible LOQ.
👉 Learn more about the downstream impact of poor control in the consequences of nitrosamine detection.
3. Sample Preparation Techniques Supporting Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing
Sample preparation has a direct impact on achievable LOQ because it controls analyte recovery and matrix cleanliness. Even the most sensitive instrument cannot compensate for poor or inconsistent sample preparation. Well-designed protocols are therefore essential for ultra-low-level testing.
Solid-phase extraction (SPE) with mixed-mode cartridges is commonly used to isolate nitrosamines while removing interfering substances. This improves signal clarity and reduces ion suppression. Cartridge selection, conditioning, and elution steps must be validated for each sample type.
Isotope dilution using deuterated nitrosamine standards corrects for extraction losses and matrix variability. Automated sample preparation systems further improve reproducibility by minimizing manual handling and contamination risks. Low-temperature solvent evaporation helps preserve volatile nitrosamines and supports consistent recovery.
👉 See how validated workflows support defensible results in nitrosamine testing for pharmaceutical drugs.
| Sample Prep Step | Optimization Focus | Typical LOQ Impact |
|---|---|---|
| SPE Cartridge Selection | Mixed-mode C18/NH₂ | ↓ Matrix interference |
| Internal Standard | Deuterated nitrosamine | ↑ Quant accuracy |
| Solvent Evaporation | Low-temp nitrogen stream | ↓ Loss of volatile NAs |
4. Chromatographic Separation for Isomer Resolution at Ultra-Low LOQ
Strong chromatographic separation is essential for maintaining ultra-low LOQs, especially when nitrosamine isomers or structurally similar compounds are present. Poor separation increases baseline noise and reduces confidence in quantitation.
Longer C18 columns, such as 150 mm × 2.1 mm with small particle sizes, improve peak resolution and retention stability. Optimized gradients, often ranging from 5–40% acetonitrile, allow baseline separation within a reasonable run time while maintaining sensitivity.
Column temperature control between 35–40°C ensures consistent retention times across batches. Dual-mode LC approaches, combining reverse-phase and HILIC, expand coverage for both polar and non-polar nitrosamines. In many cases, chromatographic quality has a greater influence on LOQ stability than detector selection.
👉 Explore cutting-edge separation strategies in emerging technologies for nitrosamine testing.
5. Calibration Strategy and Validation for Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing
Calibration is the quantitative backbone of ultra-low LOQ determination. Accurate and well-designed calibration curves ensure reliable measurement at the lowest concentration levels. Weak calibration practices can undermine otherwise strong analytical methods.
Best practice includes at least eight calibration points spanning 0.01 to 100 ng/mL. Weighted regression models, such as 1/x², help maintain accuracy at low concentrations while controlling bias at higher levels. A signal-to-noise ratio of at least 10:1 must be demonstrated at the LOQ.
Quality control samples at low, medium, and high levels should be included in every batch. Validation aligned with international guidelines ensures that results are acceptable for regulatory review and long-term use.
Validation aligned with international guidelines ensures that results are acceptable for regulatory review.
👉 Stay compliant by aligning with global guidelines for nitrosamine testing.
| Validation Parameter | Acceptance Criterion | Compliance Basis |
|---|---|---|
| LOQ Precision | %RSD ≤ 20% | ICH Q2(R2) |
| Accuracy | 80–120% recovery | EMA Bioanalytical Method Guidelines |
| S/N Ratio | ≥10:1 | Analytical Validation Standard |
6. Matrix Effects and Suppression Mitigation
Matrix effects remain a major challenge when targeting ultra-low LOQs. Co-eluting matrix components can suppress or enhance analyte signals, leading to inaccurate results. Understanding and managing these effects is critical for reliable testing.
Post-extraction spike experiments help measure the extent of suppression or enhancement. Matrix-matched calibration curves further align analytical responses between standards and samples. Together, these approaches improve consistency and data reliability.
Additional tools such as desalting steps, guard columns, and optimized injection sequences reduce cumulative matrix buildup. Combining isotope dilution with HRMS filtering can reduce matrix effects by more than 40%, supporting stable ultra-low LOQ performance. 👉 Gain deeper insight into practical solutions for overcoming matrix effects in LC-MS/MS.
7. Data Integrity, Traceability, and Reproducibility
Regulatory confidence in Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing depends heavily on data integrity and traceability. Authorities expect complete transparency and control throughout the analytical process. High-quality data is just as important as high sensitivity.
Audit trails compliant with 21 CFR Part 11 ensure accountability and inspection readiness. Digitally secured raw data files protect against unauthorized changes. Certified reference materials sourced from approved suppliers further strengthen analytical traceability.
Inter-laboratory comparison studies provide independent confirmation of reproducibility. Consistent results across multiple laboratories support ultra-low LOQ claims and strengthen regulatory submissions.
👉 Understand how structured evaluations support submissions in this nitrosamine risk assessment guide.
8. Continuous Method Improvement and Regulatory Alignment
Ultra-low LOQs must be maintained over time as regulations and technologies evolve. Routine method requalification ensures ongoing compliance with FDA, EMA, and Health Canada expectations. Proactive improvement prevents performance drift.
Annual sensitivity checks confirm that instruments continue to meet LOQ targets. Software upgrades introduce improved HRMS algorithms that enhance selectivity and data processing. Cross-validation with techniques such as GC-MS adds another level of confidence.
Participation in ISO 17025 proficiency testing programs demonstrates external competence. Continuous improvement in these areas builds long-term scientific and regulatory credibility.
👉 For regulatory-specific expectations, review nitrosamine testing requirements in Canada.
Conclusion
Achieving Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing requires a balanced strategy that integrates optimized instrumentation, strict contamination control, advanced sample preparation, and strong data governance. Each element supports the others, creating a reliable and defensible analytical system.
Laboratories that successfully apply these practices stand out as leaders in trace-level nitrosamine analysis. Their ability to deliver consistent ultra-low LOQs reflects both technical excellence and regulatory readiness. Continuous improvement remains essential to sustaining this performance.
For expert consultation or method validation support, connect with our specialists:
👉 Contact ResolveMass Laboratories Inc.
FAQs on Ultra-Low Limit of Quantitation (LOQ) in Nitrosamine Testing
The LOQ, or limit of quantitation, is the lowest concentration of a substance that can be measured with acceptable accuracy and precision. At this level, the result is not just detectable but can be reliably reported. LOQ is established through method validation studies. It ensures confidence in numerical results at low concentrations.
LOD stands for limit of detection, which is the smallest amount of an analyte that can be detected but not accurately measured. LOQ stands for limit of quantitation, where the analyte can be measured with reliable accuracy and precision. LOD confirms presence, while LOQ confirms measurable quantity. Both are key method performance indicators.
LOQ is used to define the lowest level at which laboratory results can be quantitatively trusted. It helps laboratories decide whether a reported value is reliable for regulatory, quality, or safety decisions. LOQ is especially important in trace-level testing. It supports consistent data interpretation.
In lab reports, LOQ indicates the minimum concentration that can be accurately measured by the method. Results below the LOQ are usually reported as estimated or not quantifiable. This helps prevent overinterpretation of uncertain data. LOQ adds clarity and transparency to analytical results.
A lower LOQ is generally better because it allows measurement of smaller amounts of a substance. This is especially important for impurities, contaminants, or regulated compounds. However, the LOQ must still meet accuracy and precision requirements. Extremely low LOQs without validation can be misleading.
LOQ is commonly calculated using the standard deviation of the response and the slope of the calibration curve. A typical formula is LOQ = 10 × (standard deviation ÷ slope). This approach links method variability with sensitivity. Actual calculation methods may vary by guideline.
LOD is always lower than LOQ. LOD represents the smallest detectable signal, while LOQ represents the smallest reliably measurable concentration. A substance can be detected below the LOQ but not accurately quantified. This distinction is important for correct data reporting.
LOD indicates whether an analyte is present above background noise. A result above the LOD confirms detection but does not guarantee accuracy. Values near the LOD should be interpreted with caution. LOD is mainly used for screening and presence confirmation.
LOQ ensures that reported results are accurate, precise, and scientifically defensible. It improves confidence in low-level measurements and supports regulatory compliance. LOQ also helps standardize reporting across laboratories. Overall, it strengthens data quality and decision-making.
Reference
- James, M., & Edge, T. (2021, July 12). Low-level determination of mutagenic nitrosamine impurities in drug substances by LC–MS/MS. LCGC Europe. https://www.chromatographyonline.com/view/low-level-determination-of-mutagenic-nitrosamine-impurities-in-drug-substances-by-lc-ms-ms
- Manchuri, K. M., Shaik, M. A., Gopireddy, V. S. R., Sultana, N., & Gogineni, S. (2024). Analytical methodologies to detect N-nitrosamine impurities in active pharmaceutical ingredients, drug products and other matrices. Chemical Research in Toxicology, 37(9), 1456–1483. https://doi.org/10.1021/acs.chemrestox.4c00234
- Attaluri, V. R. R. (2024, April 23). Safeguarding purity under pressure: Detecting nitrosamine contamination. European Pharmaceutical Review. https://www.europeanpharmaceuticalreview.com/article/227079/safeguarding-purity-under-pressure-detecting-nitrosamine-contamination/
- Williams, D. E., Harvey, R. G., & Sayer, J. M. (2002). Mechanisms of nitrosamine carcinogenesis. Chemical Research in Toxicology, 15(1), 2–9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12655250/
- Satla, S. R., & Gunda, R. (2025). A sensitive green analytical LC-MS/MS method for the quantification of trace levels of nine nitrosamine impurities in Zaltoprofen bulk drug. Journal of Applied Pharmaceutical Science. Advance online publication. https://doi.org/10.7324/JAPS.2026.244232


