Share with your Network:
Summary of the Article
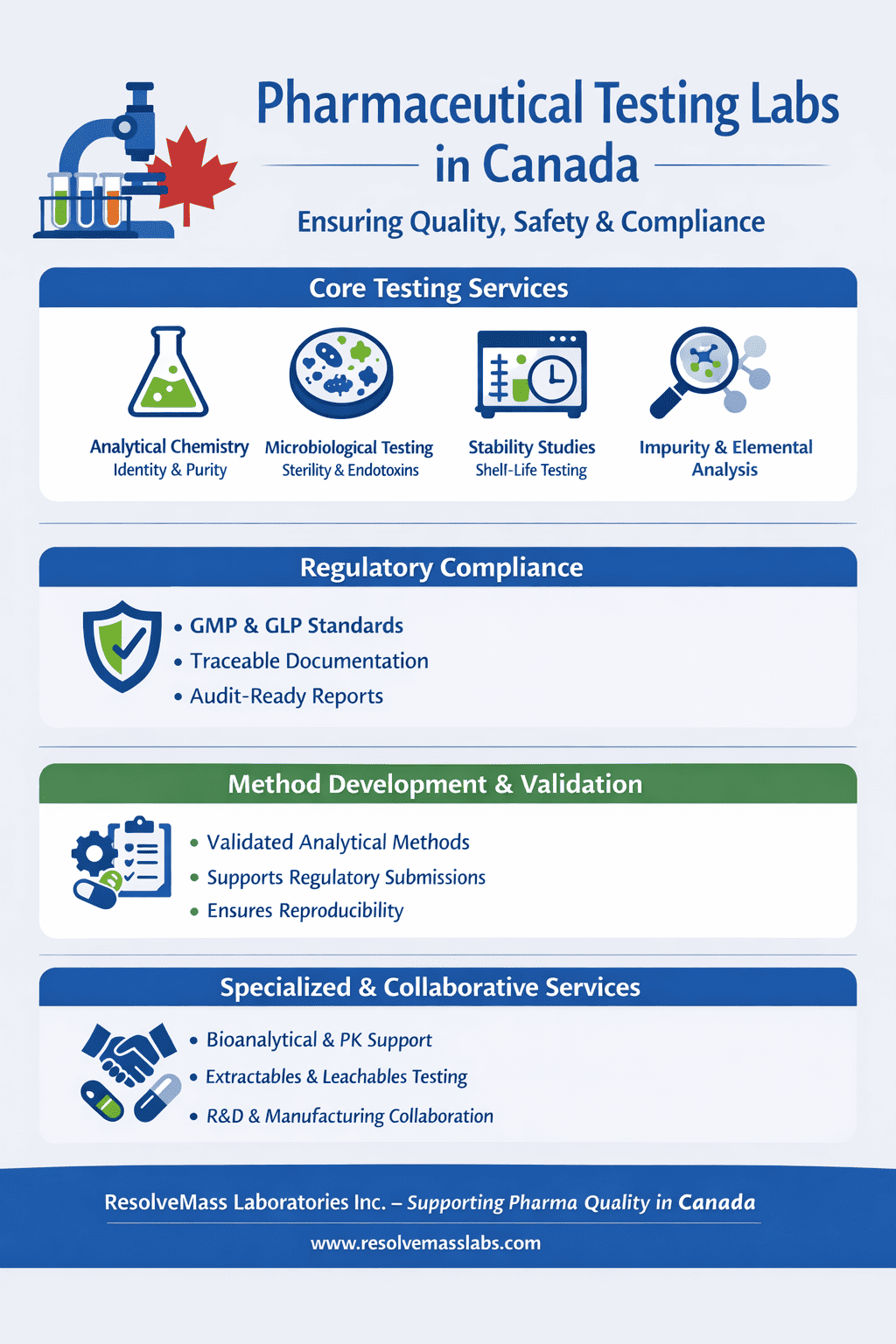
- A pharmaceutical testing laboratory in Canada ensures drug products meet national and international quality, safety, and efficacy standards.
- These labs perform chemical, microbiological, and stability testing under strict Good Manufacturing Practices (GMP) compliance.
- They play a vital role in regulatory submissions to Health Canada and global markets.
- Advanced labs also perform method validation, impurity profiling, dissolution studies, and stability testing for both APIs and finished products.
- Collaboration with R&D, manufacturers, and regulatory bodies ensures the integrity, traceability, and compliance of pharmaceutical products.
- Learn how laboratories like ResolveMass Laboratories Inc. maintain excellence and trust in Canada’s tightly regulated pharma ecosystem.
Introduction
A Pharmaceutical Testing Laboratory in Canada is much more than a basic quality control facility. It is a key pillar of regulatory compliance, patient safety, and scientific verification within the Canadian pharmaceutical industry. Every medicine sold in Canada must pass through rigorous testing before it can be approved.
These laboratories conduct detailed analytical, microbiological, and stability evaluations following Health Canada’s GMP requirements. Their work ensures that pharmaceutical products meet defined specifications from production to the end of shelf life.
Beyond routine testing, pharmaceutical laboratories help bridge the gap between drug development and commercial launch. They convert scientific research into compliant, market-ready products by generating accurate and reliable data using advanced analytical platforms such as high-resolution systems designed to capture complex molecular profiles—discover how precision-driven laboratories support this through advanced HRMS analytical solutions.
By maintaining validated methods, strict quality systems, and clear documentation, these laboratories protect public health and help manufacturers meet both Canadian and global regulatory expectations.
1. Ensuring Compliance with Health Canada’s GMP and GLP Standards in a Pharmaceutical Testing Laboratory in Canada
Every Pharmaceutical Testing Laboratory in Canada operates under Health Canada’s Division 2 GMP requirements and follows Good Laboratory Practice (GLP) principles. These rules define how laboratory processes are designed, monitored, and documented.
Strict environmental controls, validated test methods, and strong documentation systems ensure that results are accurate and reproducible. This level of control is critical during regulatory inspections and product approvals.
Compliance systems also ensure full traceability, from sample receipt to final report issuance. Each activity is recorded, reviewed, and approved to prevent errors or data loss.
By maintaining full GMP and GLP compliance, laboratories help manufacturers confidently face audits and regulatory submissions, reducing approval risks and delays.
Key Functions Under GMP/GLP Compliance:
- Standard Operating Procedures (SOPs): All activities follow approved and validated procedures.
- Instrument Qualification: Equipment undergoes IQ/OQ/PQ to confirm proper performance.
- Personnel Qualification: Analysts receive regular training and competency checks.
- Audit-Ready Data Integrity: ALCOA+ principles are followed to ensure trustworthy data.
| GMP/GLP Element | Description | Impact |
|---|---|---|
| Documentation Control | Maintaining traceable lab records | Ensures audit readiness |
| Environmental Monitoring | Air quality, humidity, and temperature control | Protects product integrity |
| Calibration & Maintenance | Scheduled instrument checks | Guarantees analytical reliability |
2. Analytical Chemistry: The Core of a Pharmaceutical Testing Laboratory in Canada
Analytical chemistry forms the foundation of every Pharmaceutical Testing Laboratory in Canada. It focuses on confirming the identity, strength, purity, and overall quality of pharmaceutical materials.
Testing is performed on raw materials, APIs, intermediates, and finished dosage forms. These tests confirm that every batch meets approved specifications before release.
Advanced analytical techniques can detect even very small levels of impurities or degradation products. For high-sensitivity impurity profiling and quantitative analysis, many laboratories rely on modern platforms—learn how integrated workflows using LC-MS analytical testing enhance pharmaceutical quality control.
Analytical data also supports regulatory submissions, stability programs, and ongoing quality control throughout the product lifecycle.
Core Analytical Services:
- HPLC/UPLC Analysis: Assay testing, impurity profiling, and degradation studies.
- GC Analysis: Residual solvent and volatile compound testing.
- Spectroscopy (FTIR, UV-Vis, ICP-MS): Identity and elemental analysis.
- Dissolution Testing: Confirms drug release performance as per pharmacopeial standards.
All methods are validated according to ICH Q2(R2) guidelines to ensure accuracy, precision, and reliability.
3. Microbiological Testing in a Pharmaceutical Testing Laboratory in Canada
Microbiological testing is essential for patient safety, especially for sterile and non-sterile products. A Pharmaceutical Testing Laboratory in Canada evaluates microbial quality at every stage of the product lifecycle.
These tests ensure compliance with USP, EP, and Health Canada microbial limits. They help prevent contaminated products from reaching the market.
Testing is carried out in controlled cleanroom environments using strict aseptic techniques to minimize contamination risks.
Microbiological results are especially important for injectables, ophthalmic products, and biologics, where contamination can cause serious health issues.
Microbiology Testing Includes:
- Sterility Testing: Performed in validated aseptic isolators.
- Bioburden Analysis: Measures microbial levels before sterilization.
- Endotoxin (LAL) Testing: Detects bacterial endotoxins in injectable products.
- Microbial Identification: Uses tools like MALDI-TOF for accurate identification.
4. Stability Studies in a Pharmaceutical Testing Laboratory in Canada
Stability testing is a mandatory regulatory requirement and a key service of a Pharmaceutical Testing Laboratory in Canada. These studies evaluate how a product behaves over time under different environmental conditions.
Stability chambers simulate long-term, intermediate, and accelerated conditions according to ICH Q1A guidelines. The data supports expiry dates and storage instructions.
Testing includes physical, chemical, and microbiological evaluations at defined intervals, often supported by advanced chromatographic methods—explore how robust separation techniques like HPLC analysis contribute to reliable stability data.
These studies help manufacturers understand degradation patterns and ensure consistent product performance throughout shelf life.
Stability Testing Parameters:
- Temperature and humidity exposure
- Photostability testing
- Degradation product analysis
- Real-time and accelerated stability models
Stability data is required for New Drug Submissions (NDS) and Abbreviated New Drug Submissions (ANDS) in Canada.
5. Method Development and Validation in a Pharmaceutical Testing Laboratory in Canada
Before routine testing can begin, methods must be properly developed and validated. A Pharmaceutical Testing Laboratory in Canada ensures all methods meet scientific and regulatory standards.
Method development focuses on achieving accuracy, specificity, and robustness. Validation confirms that methods perform consistently under different conditions.
These steps reduce testing variability and improve reliability across analysts and instruments.
Validated methods are critical for regulatory submissions, product release, and lifecycle management.
Key Steps:
- Feasibility and pre-validation studies
- Method optimization
- Validation for precision, accuracy, and detection limits
- Preparation of regulatory-ready reports
6. Impurity Profiling and Elemental Analysis in a Pharmaceutical Testing Laboratory in Canada
Impurity control is a major regulatory focus due to patient safety concerns. A Pharmaceutical Testing Laboratory in Canada uses advanced tools to detect impurities at very low levels.
Both organic and inorganic impurities are evaluated in line with ICH guidelines. For volatile and semi-volatile compounds, laboratories depend on specialized instrumentation—see how targeted impurity detection is strengthened through GC-MS testing services.
Elemental analysis confirms that heavy metals and residual catalysts stay within acceptable limits.
These studies support risk assessments and ensure compliance throughout the product lifecycle.
Impurity Studies Include:
- Organic impurities and degradation products
- Inorganic impurities and heavy metals
- Residual solvent testing as per ICH Q3C
7. Bioanalytical and Pharmacokinetic Support
Pharmaceutical testing laboratories also support clinical development through bioanalytical services. These studies measure drug levels in biological samples.
Accurate results help determine pharmacokinetic parameters needed for bioequivalence and clinical trials.
Validated bioanalytical methods ensure dependable data for regulatory decisions.
These services support both non-clinical and clinical development programs.
Key Techniques:
- LC-MS/MS analysis
- Matrix effect and stability testing
- Clinical and non-clinical sample analysis
8. Quality Assurance and Regulatory Documentation
Quality assurance supports every operation within a Pharmaceutical Testing Laboratory in Canada. QA teams oversee compliance, data accuracy, and documentation quality.
Each test result is independently reviewed before release.
Strong documentation systems ensure traceability and accountability across all activities.
Effective QA builds regulatory confidence and long-term client trust.
Documentation Includes:
- Analytical test reports
- Method validation files
- OOS investigations
- Change control and CAPA records
9. Specialized Services: Extractables, Leachables & Packaging Compatibility
Modern pharmaceutical testing laboratories offer specialized testing for packaging systems.
Extractables and leachables studies identify potential risks from packaging materials and container closure systems. To proactively manage packaging-related safety concerns, manufacturers increasingly rely on comprehensive extractables and leachables testing programs.
Compatibility studies ensure packaging does not impact product quality over time.
These evaluations are essential for regulatory compliance and patient safety.
10. Collaborative Support for R&D and Manufacturing
Pharmaceutical testing laboratories work closely with R&D and manufacturing teams.
They support formulation development, process optimization, and technology transfer.
Laboratory data helps speed up development while maintaining high quality standards.
This collaboration supports innovation without compromising compliance.
Conclusion
A Pharmaceutical Testing Laboratory in Canada serves as the scientific and regulatory backbone of the pharmaceutical industry. From raw material testing to advanced stability and impurity analysis, every activity protects product quality and patient safety.
These laboratories ensure compliance with Health Canada requirements while supporting global regulatory submissions.
In a highly regulated industry, organizations like ResolveMass Laboratories Inc. stand out for their reliability, scientific excellence, and regulatory expertise.
🔗 Contact us today to learn more
FAQs on Pharmaceutical Testing Laboratories in Canada
A pharmaceutical testing laboratory in Canada ensures that medicines meet strict quality, safety, and performance standards set by Health Canada. These laboratories verify that products are suitable for patient use through scientific testing. Their work supports regulatory approvals and ongoing product compliance. This process helps protect public health across the country.
Yes, pharmaceutical testing laboratories in Canada must operate under Health Canada’s Division 2 GMP licensing framework. They are regularly inspected to confirm compliance with GMP and GLP requirements. Licensing ensures laboratories follow controlled processes and validated methods. This oversight maintains consistency and reliability in testing results.
These laboratories perform analytical chemistry, microbiological testing, stability studies, impurity profiling, and bioanalytical testing. Each test evaluates different aspects of product quality and safety. Together, they ensure pharmaceutical products meet regulatory and scientific standards. Testing supports both development-stage and commercial products.
Many laboratories provide bioanalytical and pharmacokinetic testing for clinical and bioequivalence studies. They analyze biological samples to measure drug concentration levels. This data is essential for understanding how a drug behaves in the body. Accurate results support regulatory decisions during clinical development.
Laboratories follow strict data management systems and validated workflows to maintain data integrity. Audit trails, controlled access, and regular reviews help prevent errors or data loss. Compliance with ALCOA+ principles ensures data is complete, accurate, and traceable. This builds confidence during regulatory inspections.
Reference
- Innomar Strategies. (n.d.). Regulatory services. https://www.innomar-strategies.com/our-integrated-model/regulatory-services
- CAL Laboratories. (n.d.). Pharmaceuticals.CAL Laboratories. https://cal-laboratories.com/pharmaceuticals/Cal Laboratories
- Eurofins Canada. (n.d.). Pharmaceutical testing services. Eurofins Canada. https://www.eurofins.ca/en/our-services/pharmaceutical-testing/
- Applied Pharma Laboratories. (n.d.). PAL facilities. Applied Pharma Laboratories. https://appliedpharma.ca/about/facilities/pal/


