Introduction
LC-MS/MS bioanalytical services are essential analytical solutions that enable pharmaceutical companies to accurately measure drug concentrations in biological samples during small molecule drug development. These services combine liquid chromatography separation with tandem mass spectrometry detection to provide the sensitivity, specificity, and throughput required for modern drug development programs and are a core part of end-to-end bioanalytical services.
In the competitive pharmaceutical landscape, selecting the right partner for LC-MS/MS bioanalytical services can determine the success or failure of a drug development program. From early discovery screening to regulated studies supporting IND and NDA submissions, bioanalytical data forms the backbone of regulatory decision-making. Understanding why bioanalysis is important is fundamental to managing risk, timelines, and development costs.
ResolveMass Laboratories Inc. specializes in delivering comprehensive bioanalytical laboratory services using advanced LC-MS/MS platforms. Our expertise spans drug development bioanalytical services from discovery through late-stage clinical trials.
Summary
LC-MS/MS bioanalytical services provide critical support for small molecule drug development through sensitive, accurate quantification of drug compounds and metabolites in biological matrices. This article explores how these advanced analytical services accelerate pharmaceutical development from discovery through clinical trials.
Key Takeaways:
- LC-MS/MS bioanalytical services offer unparalleled sensitivity (pg/mL to ng/mL range) for small molecule quantification
- Method development and validation follow FDA, EMA, and ICH regulatory guidelines ensuring data integrity
- Regulatory-compliant bioanalytical method development and method validation ensure data integrity
- Applications span PK/PD bioanalysis, toxicokinetic bioanalysis, and clinical trials
- ResolveMass Laboratories delivers comprehensive bioanalytical support with state-of-the-art instrumentation
- Regulatory compliance and quality assurance are fundamental to successful drug development programs
- Strategic bioanalytical outsourcing reduces timelines and cost risk
- ResolveMass delivers scalable, regulated bioanalytical services
1: What Are LC-MS/MS Bioanalytical Services?
LC-MS/MS bioanalytical services are specialized laboratory services that use liquid chromatography–tandem mass spectrometry to quantify drugs, metabolites, and biomarkers in biological matrices. These services are foundational to both small molecule quantification and comparative studies such as small molecule vs large molecule bioanalysis.
Core Components of LC-MS/MS Services
The bioanalytical workflow encompasses several critical stages:
- Sample Collection and Processing: Proper handling of biological matrices to preserve analyte stability
- Sample Extraction: Extraction strategies addressing bioanalytical matrix effects
- Chromatographic Separation: Resolving analytes from interfering substances
- Mass Spectrometric Detection: Tandem MS detection including LC-MS/MS bioanalysis of xenobiotics
- Data Analysis and Reporting: Converting raw instrument data into actionable pharmacokinetic parameters
Why LC-MS/MS for Small Molecules?
LC-MS/MS technology offers distinct advantages for small molecule analysis:
- Unmatched Sensitivity: Detection limits in the picogram per milliliter range enable studies with minimal sample volumes
- High Specificity: Tandem mass spectrometry provides molecular-level identification, reducing false positives
- Wide Dynamic Range: Quantification across 4-5 orders of magnitude in a single analysis
- Multiplexing Capability: Simultaneous measurement of parent drugs and multiple metabolites
- Rapid Analysis: High-throughput screening of hundreds of samples per day
For biologics programs, alternative workflows such as large molecule bioanalysis or LC-MS for large molecules may be required.
2: Applications in Small Molecule Drug Development
2.1 Preclinical Development and ADME Studies
LC-MS/MS bioanalytical services support early-stage discovery and regulated preclinical studies, bridging discovery vs regulated bioanalysis. These early-stage investigations identify optimal candidates for clinical advancement.
Critical preclinical applications include:
- In Vitro ADME Screening: Metabolic stability, protein binding, and permeability assessments
- Pharmacokinetic Studies: Plasma concentration-time profiles in animal models
- Tissue Distribution: Quantifying drug concentrations across different organs and tissues
- Metabolite Identification: Characterizing biotransformation pathways
- Drug-Drug Interaction Studies: Evaluating potential interactions with co-administered medications
These studies are often managed through an experienced bioanalytical CRO to ensure scalability.
2.2 Toxicokinetic Analysis
Toxicokinetic (TK) studies run parallel to toxicology assessments, providing exposure data that correlates with safety findings. LC-MS/MS services enable accurate determination of systemic exposure levels in toxicology species, supporting dose selection for first-in-human studies and regulatory submissions.
2.3 Clinical Trial Support
Bioanalytical services for clinical trials provide the pharmacokinetic data required to establish safe and effective dosing regimens in humans. From Phase I through Phase III, LC-MS/MS analysis delivers critical exposure information.
| Clinical Phase | Bioanalytical Objectives | Sample Types |
|---|---|---|
| Phase I | Safety, tolerability, PK characterization | Plasma, urine, sometimes CSF |
| Phase II | Dose-ranging, PK/PD relationships | Plasma, specific tissues via biopsy |
| Phase III | Confirmatory PK, special populations | Plasma, urine, pediatric samples |
| Phase IV | Post-marketing surveillance, long-term safety | Plasma, real-world sample matrices |
Clinical bioanalytical services rely heavily on LC-MS/MS for Phase I–IV trials.
3: Method Development and Validation for LC-MS/MS Bioanalytical Services
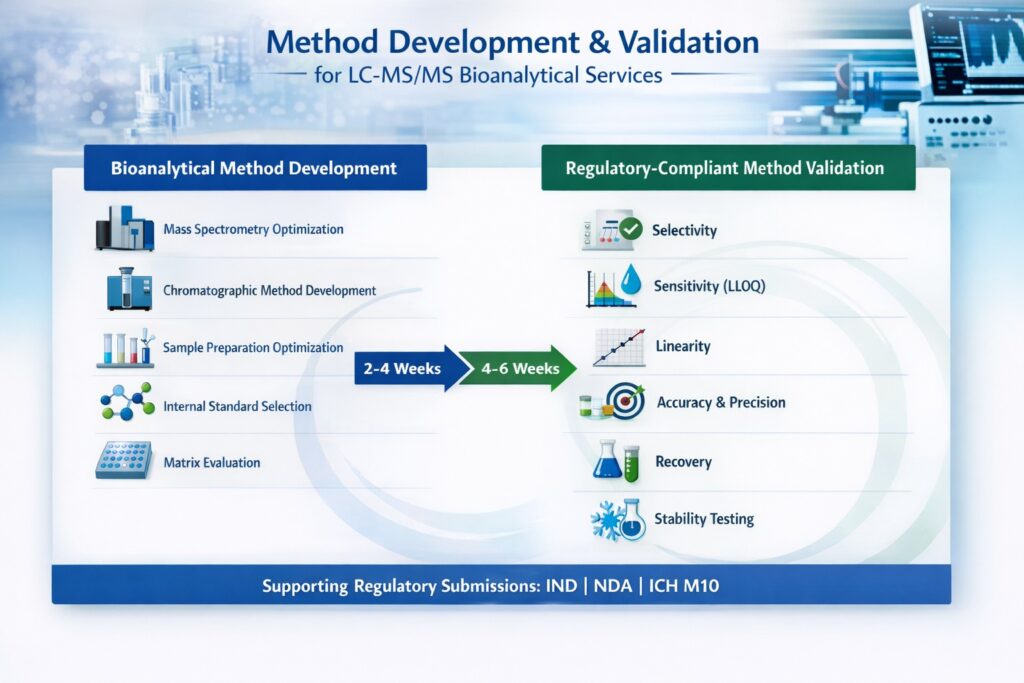
3.1 Bioanalytical Method Development Process
Method development for LC-MS/MS bioanalytical services involves optimizing every aspect of the analytical workflow to achieve reliable quantification within the required concentration range. The development process typically requires 2-4 weeks depending on compound complexity and matrix challenges.
Effective LC-MS/MS bioanalysis begins with robust method development while proactively addressing challenges in bioanalytical method development.
Key development activities include:
- Mass Spectrometry Optimization: Tuning instrument parameters for maximum sensitivity
- Chromatographic Method Development: Selecting appropriate columns and mobile phases for separation
- Sample Preparation Optimization: Evaluating extraction techniques (protein precipitation, liquid-liquid extraction, solid-phase extraction)
- Internal Standard Selection: Choosing appropriate isotopically-labeled or structural analogs
- Matrix Evaluation: Testing method performance across different biological matrices
3.2 Regulatory-Compliant Method Validation
Bioanalytical method validation ensures that analytical methods produce reliable, reproducible data suitable for regulatory decision-making. ResolveMass Laboratories follows FDA Bioanalytical Method Validation Guidance, EMA guidelines, and ICH M10 recommendations.
Bioanalytical method validation confirms assay reliability under FDA, EMA, and ICH M10 guidelines.
Validation parameters assessed include:
- Selectivity: Absence of interference from endogenous matrix components
- Sensitivity: Lower limit of quantification (LLOQ) determination
- Linearity: Calibration curve performance across the analytical range
- Accuracy and Precision: Within-run and between-run performance
- Recovery: Extraction efficiency from biological matrices
- Matrix Effects: Ion suppression or enhancement evaluation
- Stability: Bench-top, freeze-thaw, and long-term storage stability
Validation typically requires 4-6 weeks for full completion, producing a comprehensive validation report that supports regulatory submissions.
4: Regulatory Compliance and Quality Assurance
4.1 GLP and GMP Compliance
LC-MS/MS bioanalytical services for regulatory submissions must operate under Good Laboratory Practice (GLP) or Good Manufacturing Practice (GMP) guidelines to ensure data integrity and traceability. Compliance encompasses standard operating procedures, quality control systems, documentation practices, and audit readiness.
LC-MS/MS bioanalytical services supporting regulatory submissions must comply with GLP bioanalytical services standards.
ResolveMass Laboratories maintains rigorous quality systems including:
- Validated computerized systems for data management
- Temperature-monitored sample storage facilities
- Comprehensive chain of custody procedures
- Regular equipment calibration and maintenance
- Staff training and qualification programs
- Internal quality control sample analysis
- Independent quality assurance review
4.2 Documentation and Audit Trails
Complete documentation is essential for regulatory acceptance. Every analytical run generates detailed records including:
- Raw instrument data files
- Sample preparation worksheets
- Calibration curve performance metrics
- Quality control sample results
- Analyst identification and timestamps
- Deviations and corrective actions
5: Selecting the Right LC-MS/MS Bioanalytical Service Provider
Critical Evaluation Criteria
When choosing LC-MS/MS bioanalytical services for your small molecule program, consider these essential factors:
Technical Capabilities:
- Range of analytical platforms (UPLC-MS/MS, conventional LC-MS/MS)
- Sensitivity specifications for your compound class
- Experience with your specific biological matrices
- Multiplexing capabilities for parent drug and metabolites
Regulatory Experience:
- Track record with FDA, EMA submissions
- GLP/GMP compliance status
- Successful regulatory inspections
- Understanding of global regulatory requirements
Operational Excellence:
- Realistic turnaround time commitments
- Communication protocols and reporting frequency
- Capacity for both routine and rush analyses
- Sample storage and retention capabilities
Scientific Expertise:
- Ph.D.-level method development scientists
- Problem-solving capability for challenging compounds
- Publications and presentations in bioanalytical field
- Collaborative approach to study design
Sponsors should evaluate:
- Proven bioanalytical CRO services for PK and TK
- Experience in bioanalytical services in North America
- Transparent bioanalytical testing services cost
- Capability to support biosimilar bioanalysis and cell and gene therapy bioanalysis
6: Advantages of Partnering with ResolveMass Laboratories
ResolveMass Laboratories Inc. delivers comprehensive LC-MS/MS bioanalytical services designed specifically for small molecule drug development programs. Our commitment to scientific excellence, regulatory compliance, and client partnership ensures your program receives the analytical support necessary for success.
ResolveMass Laboratories Inc. offers a complete bioanalytical services overview with specialized support for biotech and pharma.
Our Differentiators:
- Affordable solutions for biotech startups
- Expertise supporting bioanalytical CRO for drug discovery
- Flexible bioanalytical services outsourcing for pharma
- Advanced bioanalytical quantification strategies
7: Cost Considerations and Timeline Planning
Bioanalytical Service Pricing Factors
Understanding cost drivers helps optimize your bioanalytical budget:
- Method Development Complexity: Novel compounds or challenging matrices require more development time
- Validation Scope: Full validation versus partial or cross-validation impacts costs
- Sample Volume: Per-sample costs decrease with larger study sizes
- Matrix Type: Plasma and serum are most economical; tissues and special matrices increase costs
- Turnaround Requirements: Standard timelines are most cost-effective; rush services carry premiums
Typical Project Timelines
Method Development: 2-4 weeks Full Validation: 4-6 weeks Clinical Sample Analysis (per batch of 100-200 samples): 1-2 weeks Study Report Generation: 1-2 weeks post-analysis completion
Planning bioanalytical activities into your overall program timeline ensures efficient progression through development milestones.
8: Future Trends in LC-MS/MS Bioanalytical Services
The bioanalytical field continues evolving with technological advances and regulatory changes:
- Microsampling Technologies: Reduced sample volumes enabling pediatric and frequent sampling studies
- High-Resolution Mass Spectrometry: Enhanced selectivity for complex metabolite profiling
- Automated Sample Preparation: Increased throughput and reduced variability
- Cloud-Based Data Systems: Enhanced collaboration and real-time data access
- Artificial Intelligence: Predictive method development and automated data review
ResolveMass Laboratories continually invests in emerging technologies to provide clients with cutting-edge analytical capabilities.
Conclusion
LC-MS/MS bioanalytical services are indispensable for successful small molecule drug development, providing the sensitive, accurate, and regulatory-compliant quantification required from discovery through commercialization. The selection of an experienced, quality-focused bioanalytical partner significantly impacts development timelines, regulatory success, and ultimately, the ability to bring new therapies to patients who need them.
ResolveMass Laboratories Inc. delivers scientifically rigorous, regulatory-aligned LC-MS/MS bioanalytical services through a collaborative CRO model. Whether you are planning to outsource bioanalysis for biotech startups or manage large clinical programs, our team provides the expertise, transparency, and reliability required to move your drug development program forward.
Whether you’re advancing a first-in-class molecule through preclinical development or supporting a pivotal Phase III trial, our team stands ready to provide the LC-MS/MS bioanalytical services that drive your program forward.
Frequently Asked Questions:
LC-MS is used in drug development to identify, quantify, and characterize drugs, metabolites, and biomarkers across discovery, preclinical, and clinical stages.
Key applications include:
-Pharmacokinetics (PK): Measuring drug concentration–time profiles
-Toxicokinetics (TK): Correlating exposure with toxicity
-ADME studies: Absorption, distribution, metabolism, and excretion analysis
-Bioavailability and bioequivalence: Comparing formulations
-Metabolite identification: Characterizing metabolic pathways
-Clinical trial bioanalysis: Supporting Phase I–IV studies
-Biomarker quantification: Measuring pharmacodynamic markers
LC-MS and LC-MS/MS are considered the gold standard for small molecule bioanalysis due to their sensitivity and selectivity.
An LC-MS drug test is a laboratory analytical test that uses liquid chromatography coupled with mass spectrometry to detect and quantify drugs and their metabolites in biological samples.
Common features of an LC-MS drug test:
-High sensitivity (pg/mL–ng/mL range)
-High specificity with minimal false positives
-Ability to confirm drug identity and concentration
-Used in pharmaceutical research, clinical trials, toxicology, and forensic testing
Unlike immunoassays, LC-MS drug tests provide definitive identification based on molecular mass.
LC-MS/MS tests for drugs, metabolites, impurities, xenobiotics, and biomarkers in complex biological matrices.
LC-MS/MS can detect:
-Small-molecule drug compounds
-Active and inactive metabolites
-Endogenous biomarkers
-Environmental and foreign chemicals (xenobiotics)
-Trace-level compounds in plasma, serum, urine, or tissues
Tandem mass spectrometry (MS/MS) adds an extra level of selectivity, making it ideal for regulated bioanalytical studies.
The basic principle of LC-MS/MS is the separation of compounds by liquid chromatography followed by mass-based detection and quantification using two stages of mass spectrometry.
Step-by-step principle:
-Liquid chromatography (LC): Separates compounds based on chemical properties
-Ionization: Converts analytes into charged ions (commonly ESI or APCI)
-First mass analyzer (MS1): Selects the target parent ion
-Collision cell: Fragments the parent ion
-Second mass analyzer (MS2): Detects specific product ions for quantification
This process provides high sensitivity and specificity, even in complex biological samples.
The four main types of chromatography are classified based on the separation mechanism between the stationary and mobile phases.
The four types are:
-Liquid Chromatography (LC): Separation based on polarity and interaction with the stationary phase
-Gas Chromatography (GC): Separation of volatile compounds in the gas phase
-Thin-Layer Chromatography (TLC): Rapid qualitative separation on a coated plate
-Ion-Exchange Chromatography: Separation based on ionic charge
In pharmaceutical bioanalysis, liquid chromatography (LC) is most commonly paired with mass spectrometry.
Reference
- Small molecule biomarker discovery: Proposed workflow for LC-MS-based clinical research projects.https://www.sciencedirect.com/science/article/pii/S2667145X2300010X
- Development and validation of an LC–MS/MS generic assay platform for small molecule drug bioanalysis.https://www.sciencedirect.com/science/article/abs/pii/S073170852100296X
- Bioanalytical strategies in drug discovery and development.https://www.tandfonline.com/doi/abs/10.1080/03602532.2021.1959606
- Applications of LC/MS in structure identifications of small molecules and proteins in drug discovery.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/full/10.1002/jms.1184

