Introduction
Virtual biotech companies are revolutionizing pharmaceutical development through asset-light business models that prioritize agility and innovation over infrastructure. A bioanalytical CRO for virtual biotech serves as the critical scientific backbone that enables these companies to advance drug candidates through preclinical and clinical development without establishing costly in-house laboratories. By strategically partnering with organizations offering comprehensive bioanalytical services, virtual biotechs gain access to state-of-the-art analytical capabilities, regulatory expertise, and scientific talent that would otherwise require millions in capital investment.
The pharmaceutical landscape has shifted from vertically integrated models to distributed ecosystems where execution is outsourced to experts. In this environment, specialized bioanalytical CROs have become indispensable partners across discovery, IND-enabling, and clinical phases.
Summary
Virtual biotech companies successfully build bioanalytical capabilities by partnering with specialized contract research organizations (CROs) rather than establishing in-house laboratories. This strategic approach delivers several key advantages:
- Cost efficiency through bioanalytical outsourcing
- Rapid development via regulated bioanalytical services
- Access to LC-MS/MS bioanalytical services
- Scalable support for small and large molecule quantification
- Regulatory readiness for IND and NDA submissions
Partnering with a bioanalytical CRO for virtual biotech companies is not just a cost-saving measure—it’s a strategic imperative that enables lean operations while maintaining scientific rigor throughout drug development.
1: Understanding the Virtual Biotech Model
What Defines a Virtual Biotech Company?
Virtual biotech companies operate with minimal physical infrastructure and lean internal teams, outsourcing most technical and operational functions to specialized service providers. These companies typically maintain fewer than 10-20 full-time employees who focus on strategic direction, intellectual property management, regulatory strategy, and partnership coordination. The virtual model emerged as a response to the astronomical costs and lengthy timelines associated with traditional pharmaceutical development.
Virtual biotech companies operate with minimal infrastructure, relying heavily on outsourced bioanalytical laboratory services. Internal teams focus on strategy, IP, and regulatory oversight while execution is delegated to expert CROs.
Why Virtual Biotechs Need Bioanalytical CRO Partners
Bioanalytical testing is complex, expensive, and requires specialized infrastructure that virtual companies cannot justify building. Bioanalytical services encompass method development, validation, sample analysis, pharmacokinetics, immunogenicity testing, and biomarker analysis—all requiring sophisticated instrumentation like LC-MS/MS systems, immunoassay platforms, and flow cytometry equipment. A single LC-MS/MS system can cost $500,000-$1,000,000, and that’s before considering facility requirements, maintenance, qualified personnel, and regulatory compliance infrastructure.
Bioanalysis requires advanced platforms such as high-throughput bioanalysis, validated workflows, and compliance with GLP standards. These capabilities are impractical to build internally.
Virtual biotechs recognize that partnering with a bioanalytical CRO for virtual biotech needs delivers several strategic advantages:
- Immediate access to GLP bioanalytical services
- Established regulatory track record
- Flexible capacity without long-term commitments
- Risk sharing in method development
- Access to diverse technology platforms
- Expertise in bioanalytical quantification
- Cost transparency via affordable bioanalytical services for biotech startups
2: Core Bioanalytical Capabilities Virtual Biotechs Need
2.1 Essential Services from a Bioanalytical CRO
Virtual biotech companies require comprehensive bioanalytical support across the drug development continuum. The most critical capabilities include:
| Service Category | Key Applications | Development Phase |
|---|---|---|
| Method Development & Validation | LC-MS/MS, ELISA, ligand binding assays | Preclinical to Clinical |
| Pharmacokinetic Analysis | ADME studies, tissue distribution | IND-enabling, Phase I-III |
| Immunogenicity Testing | ADA detection, NAb assessment | Clinical trials |
| Biomarker Analysis | Target engagement, PD markers | All phases |
| Stability Testing | Drug product characterization | CMC development |
| Sample Management | Biorepository services, chain of custody | Clinical trials |
2.2 Method Development and Validation
Method development and validation form the foundation of all bioanalytical work, establishing the analytical procedures that will generate data for regulatory submissions. A qualified bioanalytical CRO for virtual biotech partners brings established platforms and experienced scientists who can develop robust, validated methods in weeks rather than months. These methods must meet stringent regulatory guidelines including FDA Bioanalytical Method Validation Guidance and EMA guidelines on bioanalytical method validation.
Robust methods underpin all bioanalytical data. CROs with expertise in bioanalytical method development and bioanalytical method validation ensure regulatory acceptance.
The validation process includes:
- Selectivity: Ensuring the method measures only the intended analyte
- Sensitivity: Establishing the lower limit of quantification (LLOQ)
- Accuracy and Precision: Demonstrating reproducible, accurate measurements
- Linearity: Confirming proportional response across the relevant concentration range
- Stability: Proving analyte stability under various conditions
They also proactively manage:
2.3 Pharmacokinetic and Pharmacodynamic Studies
Pharmacokinetic (PK) studies characterize how the body processes a drug—absorption, distribution, metabolism, and excretion. Pharmacodynamic (PD) studies assess how the drug affects the body. Virtual biotechs rely on bioanalytical CROs to generate the PK/PD data that inform dosing strategies, predict human exposure, and support regulatory submissions.
These studies require sophisticated analytical capabilities including:
- High-sensitivity LC-MS/MS for small molecule quantification
- Ligand binding assays for biologics
- Metabolite identification and characterization
- Tissue distribution studies
- Drug-drug interaction assessments
PK/PD studies are central to dose justification and safety evaluation. CROs provide:
Advanced LC-MS techniques such as LC-MS/MS bioanalysis of xenobiotics are essential for exposure assessment.
2.4 Immunogenicity and Biomarker Testing
For biotherapeutics, immunogenicity testing is critical as anti-drug antibodies (ADAs) can neutralize therapeutic effect or cause adverse reactions. Specialized bioanalytical CROs provide tiered immunogenicity testing strategies including screening, confirmation, and neutralizing antibody assays. Biomarker analysis helps virtual biotechs demonstrate target engagement, mechanism of action, and therapeutic effect—essential data for go/no-go decisions and regulatory discussions.
2.5 Small vs Large Molecule and Advanced Modalities
Virtual biotechs often work across modalities, requiring CROs experienced in:
- Small molecule vs large molecule bioanalysis ()
- Large molecule bioanalysis
- LC-MS for large molecules
- Biosimilar bioanalysis ()
- Cell and gene therapy bioanalysis
2.6 Biomarker and Immunogenicity Testing
Biomarkers support proof-of-mechanism and regulatory confidence. Specialized biomarker bioanalytical services enable:
- Target engagement analysis
- Pharmacodynamic monitoring
- Clinical decision-making
3: Strategic Advantages of CRO Partnerships for Virtual Biotechs
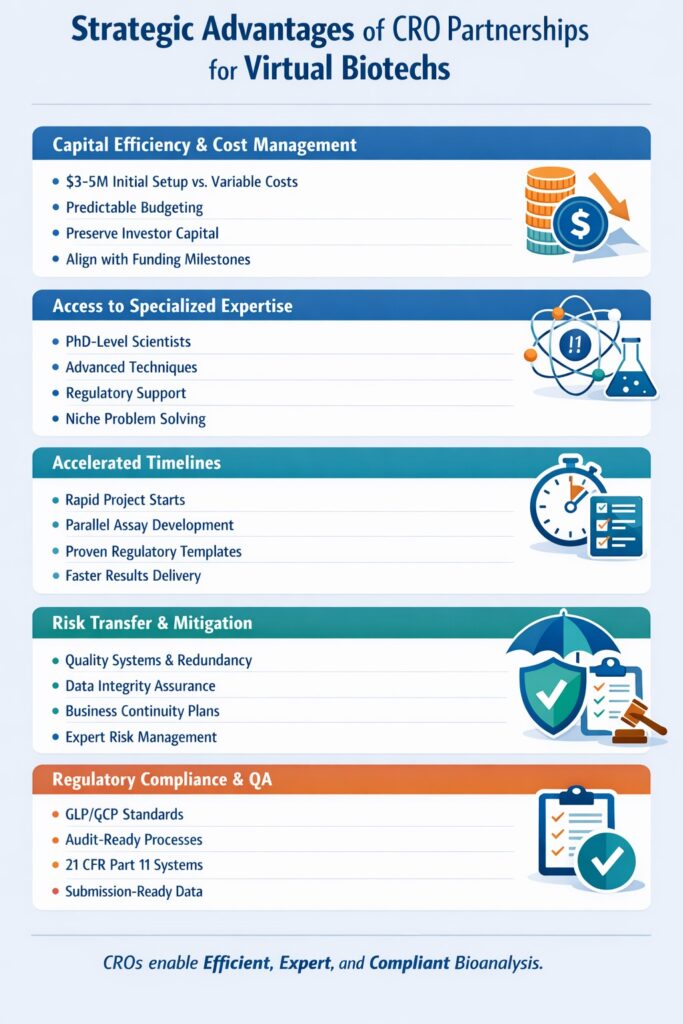
3.1 Capital Efficiency and Cost Management
Capital efficiency represents the primary driver for virtual biotech adoption of the CRO partnership model. Building an in-house bioanalytical laboratory requires $3-5 million in initial capital for equipment, facilities, and infrastructure, plus $2-3 million annually in operating costs including personnel, maintenance, consumables, and quality systems. By contrast, partnering with a bioanalytical CRO for virtual biotech converts these fixed costs into variable expenses aligned with project needs and funding cycles.
Outsourcing eliminates infrastructure investment and aligns with bioanalytical services outsourcing for pharma.
Virtual biotechs benefit from predictable costs informed by bioanalytical testing services cost models.
This financial flexibility provides several tangible benefits:
- Preservation of investor capital for core R&D activities
- Alignment of expenses with development milestones and funding rounds
- Elimination of depreciation and equipment obsolescence risk
- Reduced overhead without facility management responsibilities
- Predictable budgeting through fixed-price or time-and-materials contracts
3.2 Access to Specialized Expertise
The expertise gap between generalist scientists and specialized bioanalytical experts is substantial. Top-tier bioanalytical CROs employ PhD-level scientists with decades of experience in specific analytical techniques, therapeutic modalities, and regulatory requirements. Virtual biotechs gain immediate access to this expertise without the lengthy recruitment process, competitive salaries, and retention challenges associated with building an internal team.
Expert CRO scientists provide:
- Method troubleshooting and optimization expertise
- Regulatory strategy and submission support
- Knowledge of industry best practices
- Experience across diverse therapeutic areas
- Technical problem-solving for complex analytical challenges
3.3 Accelerated Development Timelines
Time compression is critical in drug development where every month of delay can cost millions in lost revenue and extend patent clocks. A bioanalytical CRO for virtual biotech accelerates timelines through parallel processing, established workflows, and experienced teams who can anticipate and avoid common pitfalls. Where establishing in-house capabilities might require 12-18 months, CRO partnerships can initiate work within weeks.
Timeline advantages include:
- Immediate project initiation without facility setup delays
- Concurrent method development across multiple assays
- Established regulatory templates and submission experience
- Proven quality systems eliminating validation time
- Flexible resource allocation to meet aggressive deadlines
3.4 Risk Transfer and Mitigation
Technical and operational risks pervade bioanalytical work—method failures, equipment breakdowns, staff turnover, regulatory non-compliance, and data integrity issues. By partnering with established CROs, virtual biotechs transfer many of these risks to organizations with robust quality systems, redundant equipment, deep bench strength, and insurance coverage. Reputable bioanalytical CROs maintain disaster recovery plans, business continuity procedures, and quality management systems that would be prohibitively expensive for individual virtual biotechs to replicate.
3.5 Regulatory Compliance and Quality Assurance
Regulatory compliance represents one of the most compelling reasons virtual biotechs partner with bioanalytical CROs. Established CROs operate under FDA-inspected, GLP-compliant quality systems with documented SOPs, validated computer systems, trained personnel, and comprehensive audit trails. They maintain regulatory expertise on current guidelines and have established relationships with regulatory agencies through previous submissions and inspections.
Quality assurance infrastructure includes:
- 21 CFR Part 11 compliant electronic systems
- Regular regulatory inspections demonstrating compliance track record
- Comprehensive training programs ensuring personnel competency
- Document control systems maintaining audit trails
- Change control procedures managing analytical modifications
- CAPA systems driving continuous improvement
CROs experienced in regulated vs discovery bioanalysis ensure submission-ready datasets.
Their compliance frameworks explain why bioanalysis is important in regulatory decision-making.
4: How to Select the Right Bioanalytical CRO Partner
Virtual biotechs should prioritize partners offering:
- Bioanalytical CRO services for PK and TK
- Bioanalytical CRO for drug discovery
- Regional expertise such as bioanalytical services in North America
Startups benefit greatly from CROs that actively support outsourcing bioanalysis for biotech startups.
Critical Evaluation Criteria
Selecting the wrong bioanalytical CRO for virtual biotech projects can derail development timelines and compromise data quality. Virtual biotechs should evaluate potential partners using a comprehensive framework that assesses technical capabilities, quality systems, regulatory track record, and cultural fit.
Technical Capabilities Assessment:
- Range of analytical platforms (LC-MS/MS, ELISA, flow cytometry, etc.)
- Experience with your therapeutic modality (small molecule, biologics, cell/gene therapy)
- Method development expertise and innovation capacity
- Sample throughput and scalability
- Technology investments and platform modernization
Quality and Regulatory Track Record:
- FDA/EMA inspection history and outcomes
- GLP/GMP compliance documentation
- Quality metrics (first-time validation success rate, OOS investigations, data integrity)
- Regulatory submission experience
- Client audit reports and findings
Operational Considerations:
- Geographic location and time zone compatibility
- Communication protocols and project management approach
- Sample logistics and biorepository capabilities
- Turnaround times and capacity availability
- Flexibility in study design modifications
- Pricing transparency and contract terms
Partnership Compatibility:
- Cultural alignment and shared values
- Responsiveness and accessibility
- Scientific collaboration approach
- Conflict of interest policies
- Long-term partnership potential
Red Flags to Avoid
Virtual biotechs should be alert to warning signs that indicate potential partnership problems:
- Overpromising capabilities without supporting documentation
- Lack of regulatory inspection history suggesting limited experience
- High personnel turnover indicating organizational instability
- Reluctance to provide client references or example reports
- Inflexible contracting terms that don’t accommodate startup needs
- Poor communication responsiveness during evaluation phase
- Absence of quality metrics or refusal to discuss performance
- Significantly lower pricing without clear justification
Building Effective CRO Relationships
The most successful virtual biotech-CRO partnerships transcend transactional vendor relationships to become true collaborations. Virtual biotechs can foster productive partnerships through several best practices:
Clear Communication:
- Establish regular communication cadences (weekly calls, monthly reviews)
- Define escalation paths for technical and timeline issues
- Share strategic context to help CROs anticipate future needs
- Provide timely feedback on deliverables and performance
Aligned Expectations:
- Document detailed study protocols and acceptance criteria upfront
- Agree on timelines with realistic buffers for method development challenges
- Clarify roles and responsibilities, especially for regulatory interactions
- Establish data ownership and publication rights in contracts
Strategic Engagement:
- Involve CRO scientists in early planning discussions
- Leverage CRO regulatory expertise in submission strategy
- Consider CRO input on experimental design optimization
- Treat CRO team as extension of your scientific organization
5: Future Trends in Bioanalytical CRO Partnerships
Technology Integration and Digital Innovation
The bioanalytical landscape is rapidly evolving with artificial intelligence, machine learning, and advanced automation. Progressive bioanalytical CROs are investing in these technologies to improve data quality, accelerate method development, and enhance predictive capabilities. Virtual biotechs should seek CRO partners who demonstrate commitment to innovation through:
- AI-assisted method optimization
- Automated data review and anomaly detection
- Electronic laboratory notebooks with advanced analytics
- Cloud-based data platforms for real-time access
- Integration with clinical trial management systems
Specialized Therapeutic Expertise
As novel therapeutic modalities proliferate—cell therapies, gene therapies, RNA therapeutics, antibody-drug conjugates—bioanalytical CROs are developing specialized capabilities to support these complex molecules. Virtual biotechs developing innovative therapeutics should prioritize CRO partners with demonstrated expertise in relevant modalities, including specialized assays, appropriate analytical platforms, and regulatory precedent.
Strategic Partnership Models
The relationship between virtual biotechs and bioanalytical CROs continues evolving beyond project-based engagements toward strategic partnerships. Some emerging models include:
- Dedicated capacity agreements providing guaranteed availability
- Risk-sharing arrangements with milestone-based payments
- Embedded scientist models placing CRO staff at biotech sites
- Platform technology access for proprietary analytical methods
- Co-development agreements for novel analytical approaches
As pipelines expand, CROs offering a full bioanalytical services overview will increasingly function as long-term strategic partners rather than vendors.
Conclusion
Virtual biotech companies have fundamentally reimagined pharmaceutical development by building scientific capability through strategic partnerships rather than internal infrastructure. Selecting the right bioanalytical CRO for virtual biotech operations is not merely a procurement decision—it’s a strategic imperative that directly impacts development timelines, regulatory success, and capital efficiency. The most successful virtual biotechs recognize that their CRO partners function as extensions of their scientific teams, bringing specialized expertise, regulatory experience, and operational excellence that would be impossible to replicate internally.
By leveraging end-to-end bioanalytical services in drug development, virtual biotechs can advance confidently from discovery through clinical phases while maintaining data integrity and regulatory compliance.
The transformation of drug development through virtual models and specialized partnerships demonstrates that innovation in business models can be as impactful as innovation in science itself. Virtual biotechs that master the art of CRO partnership orchestration will continue leading the next generation of pharmaceutical breakthroughs.
Frequently Asked Questions:
A CRO (Contract Research Organization) in biotech is a specialized service provider that supports drug development by conducting outsourced scientific, technical, and regulatory activities.
Biotech companies—especially virtual and early-stage firms—use CROs to access expertise in areas such as bioanalytical testing, preclinical studies, clinical trials, data management, and regulatory submissions without building in-house infrastructure.
In short: A CRO enables biotech companies to advance drug candidates faster, compliantly, and cost-effectively.
CRO collaboration is a strategic partnership between a sponsor (biotech or pharma company) and a CRO where both parties work together to execute part or all of a drug development program.
Unlike transactional outsourcing, effective CRO collaboration involves:
-Shared scientific planning
-Transparent communication
-Aligned timelines and milestones
-Joint problem-solving and risk management
For virtual biotech companies, CRO collaboration often means the CRO functions as an extension of the internal team.
The top 5 skills of a Clinical Research Coordinator (CRC) are:
-Protocol Compliance – Ensuring studies are conducted according to approved protocols and GCP
-Patient Management – Screening, consenting, scheduling, and monitoring trial participants
-Regulatory Documentation – Managing IRB submissions, essential documents, and audit readiness
-Data Accuracy and Integrity – Maintaining precise source documents and case report forms
-Communication Skills – Coordinating effectively with investigators, sponsors, CROs, and patients
A strong CRC is critical for smooth site operations and data quality in clinical trials.
Many CROs are struggling due to industry-wide operational and economic pressures, including:
-Talent shortages and high staff turnover
-Increased regulatory scrutiny and compliance costs
-Aggressive timelines from sponsors
-Margin pressure from competitive pricing
-Rapid growth in trial complexity (biologics, cell & gene therapy)
Additionally, some CROs face challenges transitioning from volume-driven models to quality- and partnership-driven models, which sponsors increasingly demand.
Neither CRC nor CRA is better overall; it depends on your career preference.
CRC is better if you prefer hands-on, site-based work with patients and minimal travel.
CRA is better if you want higher salary, career advancement, and monitoring multiple sites, with more travel.
Reference
- Scott Summerfield, Roger Hayes, Meina Liang, Afshin Safavi, Dominic Warrino, Lina Luo.The Business of Bioanalysis: New Technology Integration into Bioanalytical Workflows.https://www.tandfonline.com/doi/full/10.4155/bio-2018-0269
- Stephen Lowes.Outsourcing in Bioanalysis: A CRO Perspective.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4994
- Philip Timmerman,Matthew Barfield,Lee Goodwin,Iain Love,Kyra Cowan,Lene Anderson.Beyond contracts: principles for Pharma–CRO collaboration from the European Bioanalysis Forum.https://www.tandfonline.com/doi/full/10.1080/17576180.2025.2582422
- Xiaojing Yu &Arkady I Gusev.CRO Benchmarking for Clinical Biomarker Analysis Outsourcing.https://www.tandfonline.com/doi/full/10.4155/bio-2019-0123
- Scott G Summerfield, Christopher Evans, Neil Spooner, John A Dunn, Matthew E Szapacs &Eric Yang.Integrating Internal and External Bioanalytical Support to Deliver A Diversified Pharmaceutical Portfolio.https://www.tandfonline.com/doi/abs/10.4155/bio.14.93
- Roger Hayes.Bioanalytical Outsourcing: Transitioning from Pharma to Cro.https://www.tandfonline.com/doi/full/10.4155/bio-2017-4996

