🔍 Summary of the Article
- Analytical method development for IND and NDA submissions is central to CMC compliance and regulatory approval.
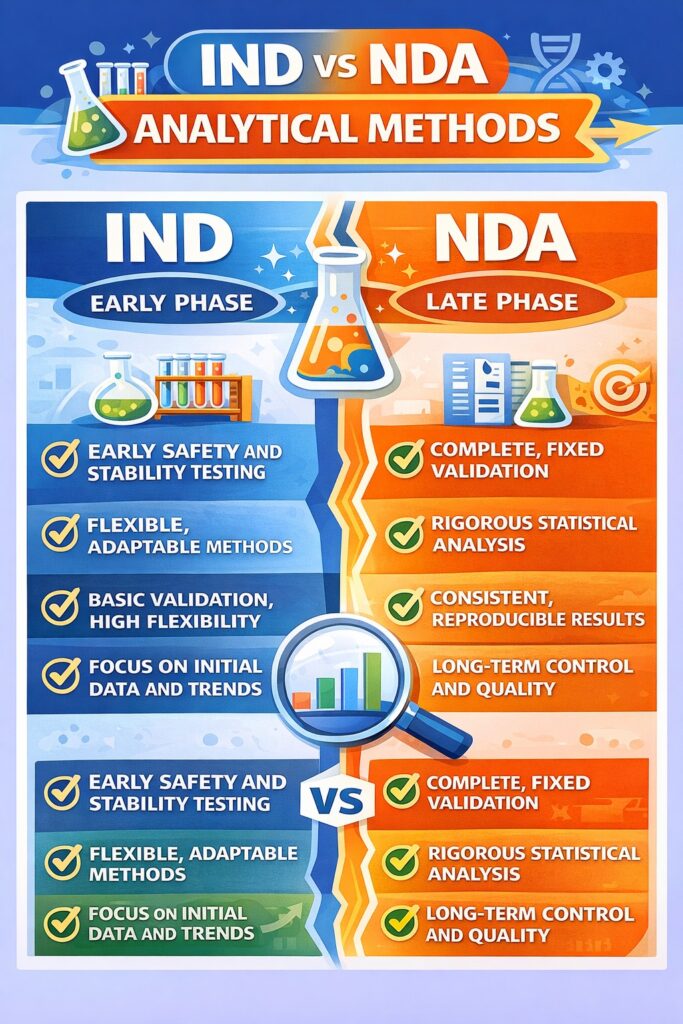
- IND phase focuses on fit-for-purpose methods ensuring safety and stability, while NDA requires fully validated methods for quality assurance and reproducibility.
- The method validation strategy, acceptance criteria, and regulatory expectations differ significantly between IND and NDA stages.
- Analytical methods evolve through phase-appropriate development, robust validation, and regulatory documentation.
- This article details critical CMC elements, validation parameters, phase-specific expectations, and FDA/ICH guidance alignment.
Introduction to Analytical Method Development for IND and NDA
Analytical Method Development for IND and NDA submissions forms the scientific backbone of Chemistry, Manufacturing, and Controls (CMC). These methods define how identity, strength, purity, and overall quality of a drug substance or drug product are measured in a reliable way. Without strong analytical methods, it is not possible to demonstrate product safety or performance.
In the IND stage, analytical methods are developed to support early clinical trials. The primary goal is to ensure patient safety while collecting meaningful data using limited manufacturing experience. These methods often change as product understanding improves.
In contrast, NDA-stage analytical methods must be highly consistent, accurate, and robust. They are used for routine release testing, stability studies, and long-term quality control. Regulators carefully review these methods and expect full validation.
The shift from IND to NDA reflects not just scientific progress, but also regulatory readiness and the organization’s ability to control analytical quality at a commercial scale.
Expert Resource: For specialized support in building a compliant framework, explore our Analytical Method Development and Validation Services.
Understanding the Role of Analytical Methods in CMC
Analytical methods within CMC ensure that both the drug substance and drug product consistently meet predefined quality standards. They provide measurable proof that the product performs as intended throughout development and commercialization.
From a regulatory perspective, expectations vary by development phase. IND submissions focus on demonstrating that the investigational product is safe for human trials. Therefore, data requirements are risk-based and scientifically justified.
NDA submissions, however, require complete control of product quality. This includes fixed specifications, validated analytical methods, and long-term monitoring strategies that support commercial distribution.
Understanding these differences helps organizations design phase-appropriate analytical strategies and avoid regulatory delays during review.
| CMC Element | IND Phase (Developmental) | NDA Phase (Validation & Commercialization) |
|---|---|---|
| Analytical Purpose | Ensure safety & preliminary stability | Ensure reproducibility & product quality |
| Method Type | Fit-for-purpose | Fully validated |
| Acceptance Criteria | Flexible, based on data trends | Fixed, validated specifications |
| Documentation | Developmental report | Full validation report with statistical analysis |
| Regulatory Expectation | Justification of method suitability | Validation per ICH Q2(R2), Q14 compliance |
Regulatory Insight: To better understand the technical shift required between filings, read our guide on IND vs NDA CMC Requirements.
Phase-Appropriate Analytical Method Development for IND
During the IND phase, analytical method development must be fit for purpose rather than fully validated. The objective is to reliably assess critical quality attributes that support patient safety and early clinical decisions.
These methods are often developed alongside formulation and process optimization. As knowledge grows, changes to methods are expected and acceptable. Over-validating methods too early can slow development and reduce flexibility.
Regulators reviewing IND submissions focus more on scientific reasoning than strict numerical criteria. As long as the method is suitable and well explained, flexibility is generally allowed.
Strong IND methods create a solid foundation for later refinement and validation during NDA preparation.
Service Spotlight: Partner with an experienced team for your early-stage development through our IND CMC Services.
Key Considerations in IND Method Development
Scientific Justification: Each analytical method must include clear reasoning for selected parameters such as column type, detection mode, and sample preparation. This shows scientific understanding and appropriateness.
Selectivity and Specificity: Methods should clearly separate the active drug from impurities, excipients, and degradation products. This is essential for interpreting early impurity and stability data.
Stability-Indicating Capability: Stress studies should demonstrate that the method can detect meaningful degradation. This supports safety during clinical use.
Matrix Interference Assessment: For complex formulations, especially biologics or combination products, potential interference from excipients must be evaluated.
Interim Validation: Limited checks for accuracy, precision, and linearity help confirm method reliability without requiring full validation.
These early decisions strongly influence the success of later NDA analytical validation.
Technical Testing: Ensure your methods are truly stability-indicating with our comprehensive Forced Degradation Studies.
Analytical Method Validation for NDA – Regulatory Expectations
Unlike IND development, Analytical Method Development for IND and NDA at the NDA stage requires full and formal validation. Methods must be proven reliable for routine manufacturing and long-term quality control.
NDA validation demonstrates that analytical results remain consistent across analysts, instruments, laboratories, and time. This level of assurance is critical for regulatory approval and inspection readiness.
Validation activities must follow approved protocols, generate complete data, and be thoroughly documented. Regulators expect alignment with international guidelines and minimal scientific uncertainty.
The final outcome is a set of robust, inspection-ready analytical methods that support product release and stability programs.
NDA Validation Must Cover
- Full compliance with ICH Q2(R2) and ICH Q14 requirements.
- Reproducibility across multiple batches, analysts, instruments, and sites.
- Defined system suitability criteria and analytical control strategies.
- Use of qualified reference standards with traceability.
- Complete statistical validation reports supporting regulatory confidence.
Typical Validation Parameters
| Parameter | Description | NDA Expectation |
|---|---|---|
| Accuracy | Closeness to true value | Recovery 98–102% |
| Precision | Repeatability and reproducibility | RSD ≤ 2% |
| Linearity | Proportional response | R² ≥ 0.999 |
| Robustness | Resistance to small variations | Minimal effect |
| Specificity | Ability to identify analyte | Confirmed via forced degradation |
Strategy Guide: Build a roadmap for your commercial filing with a customized IND CMC Strategy that anticipates NDA needs.
Bridging IND to NDA Through the Method Lifecycle Approach
The transition from IND to NDA is a gradual process supported by Analytical Method Lifecycle Management, as outlined in ICH Q14 and USP <1220>. This approach treats method development as an evolving activity rather than a single event.
As product knowledge increases and manufacturing stabilizes, analytical methods become more refined and controlled. Each phase builds on previous data, reducing risk and rework.
Lifecycle management improves regulatory alignment, inspection readiness, and post-approval flexibility.
Transition Phases
Exploratory (Early IND): Initial screening, feasibility studies, and stress testing.
Optimization (Late IND): Method refinement and intermediate validation to improve reliability.
Locking (NDA Preparation): Finalization of parameters, acceptance criteria, and documentation.
Validation (NDA Filing): Full validation across sites, analysts, and instruments.
Post-Approval (Lifecycle): Ongoing verification, monitoring, and continuous improvement.
CMC Regulatory Expectations: IND vs NDA
Regulatory agencies expect analytical data that matches the development phase. Mismatch between method maturity and submission stage often leads to questions or delays.
IND reviews focus on patient safety, basic characterization, and scientific logic. NDA reviews demand completeness, consistency, and long-term control.
Demonstrating proper evolution of Analytical Method Development for IND and NDA is essential for regulatory confidence.
| CMC Section | IND Expectation | NDA Expectation |
|---|---|---|
| Drug Substance | Characterization and impurity profiling | Comprehensive control strategy |
| Drug Product | Preliminary release tests | Full validated release and stability tests |
| Specifications | Developmental | Fixed, validated limits |
| Method Validation | Partial, fit-for-purpose | Full ICH Q2/Q14 validation |
| Documentation | Method development summary | Validation protocol, data, and reports |
Statistical Rigor in Analytical Validation
Statistical expectations differ significantly between IND and NDA stages. Early development focuses on trend understanding, while NDA validation requires strict statistical proof.
IND data analysis is exploratory, with broader variability limits supported by scientific justification. NDA submissions require detailed statistical evaluation to prove reliability.
Strong statistical treatment reduces regulatory risk and supports long-term method performance.
Statistical Expectations for NDA Include:
- ANOVA for reproducibility across analysts and laboratories.
- Regression analysis for linearity and range.
- Precision testing across multiple days and conditions.
- Measurement uncertainty and bias evaluation.
- Outlier identification with scientific justification.
Integration of Analytical Method Development for IND and NDA with QbD
Regulators increasingly expect Analytical Method Development for IND and NDA to align with Quality by Design (QbD) principles. This ensures methods are robust, risk-based, and scientifically sound.
QbD focuses on understanding factors that impact method performance rather than relying only on final results. This approach improves validation success and regulatory acceptance.
Early QbD integration reduces post-approval changes and supports lifecycle management.
QbD Alignment Includes:
- Risk assessment to identify critical method parameters.
- Design of Experiments (DoE) to define robust operating ranges.
- Clear analytical control strategies and system suitability criteria.
- Ongoing monitoring and performance trending.
Avoid Common Errors: Learn how to identify and resolve CMC Deficiencies in IND & NDA before they impact your timeline.
Common Pitfalls and Regulatory Challenges
Many organizations face challenges when transitioning analytical methods from IND to NDA. These issues often stem from limited early planning or incomplete documentation.
Common problems include unaddressed matrix effects, weak forced degradation data, and poorly defined method transfer protocols. These gaps can lead to regulatory questions during NDA review.
Inadequate documentation of method evolution is another frequent concern. Regulators expect to see how methods matured over time.
Proactively addressing these risks strengthens CMC compliance and reduces approval delays.
Specialized Oversight: Discover how our CMC CRO Services can streamline your technical documentation and laboratory transfers.
Conclusion
Across the drug development lifecycle, Analytical Method Development for IND and NDA provides the scientific proof needed to ensure product quality and patient safety. These methods support regulatory decisions from first-in-human studies to commercial approval.
The shift from flexible IND methods to fully validated NDA methods reflects growing product knowledge and regulatory responsibility. This transition must be well planned, justified, and documented.
Organizations that apply lifecycle thinking, QbD principles, and phase-appropriate validation are better positioned for efficient regulatory approval and long-term success.
To discuss advanced analytical method development and validation strategies tailored for IND and NDA compliance, connect with the scientific experts:
👉 Contact us
FAQs on Analytical Method Development for IND and NDA
For an IND, analytical methods are allowed to be flexible and fit for purpose, mainly focused on patient safety and early understanding of the product. In an NDA, methods must be fully validated, highly reproducible, and suitable for routine commercial use. The level of regulatory scrutiny is much higher at the NDA stage.
Yes, an IND method can be carried forward to the NDA stage, but it usually cannot be used as-is. The method often needs further optimization, tighter controls, and complete validation. Regulators expect clear evidence that the method is robust enough for long-term commercial testing.
Analytical method validation is guided by ICH Q2(R2) and ICH Q14, along with USP <1220> and FDA CMC guidance documents. These guidelines define how methods should be developed, validated, and managed across their lifecycle. Following them helps ensure global regulatory acceptance.
Method lifecycle management should start as early as the IND phase. Early planning allows methods to evolve smoothly as product knowledge increases. This approach reduces rework, supports NDA readiness, and simplifies post-approval changes.
Yes, FDA accepts phase-appropriate validation when it is scientifically justified. During IND development, partial or interim validation is acceptable if it supports safety and decision-making. Full validation is expected only at the NDA stage.
Reference
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas