Introduction
Advanced Bioanalytical Strategies have become a critical enabler in modern drug development as pharmaceutical pipelines increasingly shift toward complex drug modalities. In today’s landscape—dominated by biologics, oligonucleotides, antibody-drug conjugates (ADCs), peptides, and gene-based therapies—traditional analytical approaches are no longer sufficient.
At ResolveMass Laboratories Inc., advanced bioanalysis is not just about measurement—it is about understanding molecular behavior, ensuring data integrity, and enabling confident decision-making across development stages. This article explores how Advanced Bioanalytical Strategies for Complex Drug Modalities are designed, implemented, and optimized to meet scientific and regulatory expectations.
Learn more about our bioanalytical services: ResolveMass Bioanalytical Services Overview
Summary
- Advanced Bioanalytical Strategies are essential for accurately characterizing complex drug modalities such as biologics, oligonucleotides, ADCs, and gene therapies.
- Modern drug modalities demand high-resolution, highly selective analytical platforms beyond conventional small-molecule assays.
- LC-MS/MS, HRMS, hybrid ligand-binding assays, and orthogonal techniques form the backbone of advanced bioanalysis (LC-MS/MS Bioanalysis of Oligonucleotides).
- Method development must be fit-for-purpose, regulatory-aligned, and scalable from discovery to clinical phases (Bioanalytical Method Development & Validation).
- Outsourcing advanced bioanalysis to specialized CROs like ResolveMass Laboratories Inc. enables faster timelines, higher data confidence, and regulatory readiness (Outsourced Bioanalysis for Drug Development). beyond conventional small-molecule assays.
- LC-MS/MS, HRMS, hybrid ligand-binding assays, and orthogonal techniques form the backbone of advanced bioanalysis.
- Method development must be fit-for-purpose, regulatory-aligned, and scalable from discovery to clinical phases.
- Outsourcing advanced bioanalysis to specialized CROs like ResolveMass Laboratories Inc. enables faster timelines, higher data confidence, and regulatory readiness.
1: What Are Advanced Bioanalytical Strategies?
Advanced Bioanalytical Strategies are integrated, science-driven analytical approaches designed to accurately quantify, characterize, and interpret complex therapeutic molecules within biological matrices such as plasma, serum, tissue, or cerebrospinal fluid. These strategies combine state-of-the-art analytical instrumentation, fit-for-purpose assay design, and regulatory-compliant method validation (Bioanalytical Method Validation) to meet the growing complexity of modern drug modalities.
Unlike traditional bioanalysis—which often focuses solely on concentration measurement—advanced bioanalytical strategies emphasize molecular understanding, selectivity, robustness, and regulatory defensibility throughout drug development.
In practical terms, Advanced Bioanalytical Strategies go beyond basic quantification and are specifically designed to address:
- Molecular heterogeneity and multiple molecular species (Small Molecule vs Large Molecule Bioanalysis)
- Chemical and enzymatic instability in biological matrices
- Extremely low systemic exposure levels (PK/PD Bioanalysis)
- Endogenous biological interference (Bioanalytical Matrix Effects)
- The need for both quantitative and qualitative insights
These strategies ensure that generated data truly reflect the biological behavior of the drug and can reliably support decision-making.
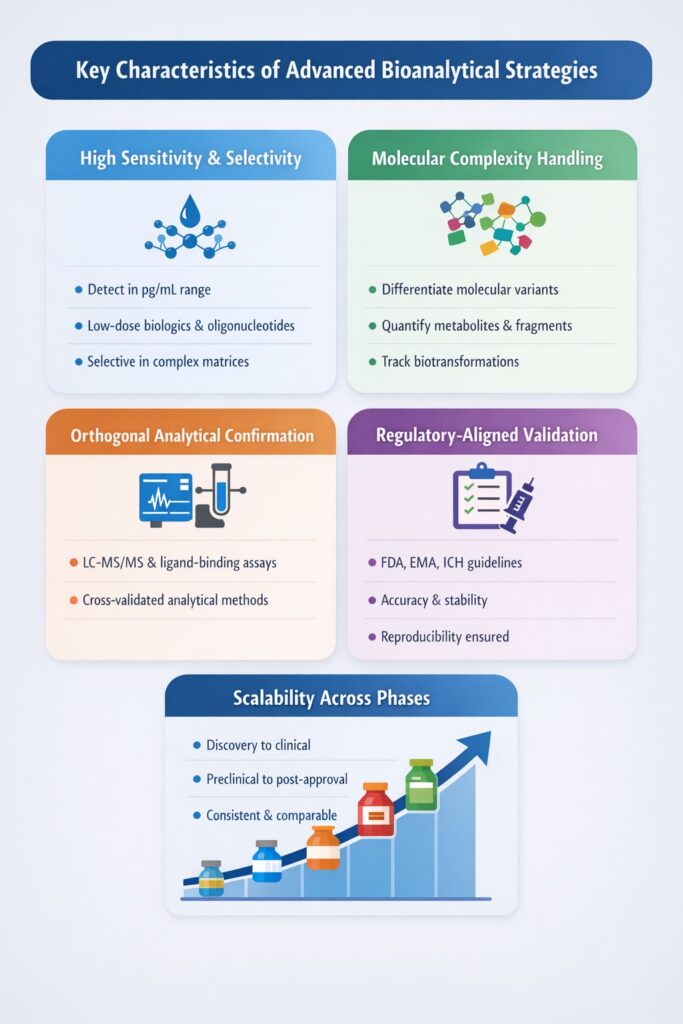
2: Key Characteristics of Advanced Bioanalytical Strategies
Advanced Bioanalytical Strategies are distinguished from conventional analytical approaches by their ability to address the scientific, technical, and regulatory challenges posed by modern drug modalities. These strategies are deliberately designed to generate high-quality, reliable, and interpretable data across complex biological systems.
2.1 High Sensitivity and Selectivity
Advanced Bioanalytical Strategies are engineered to detect and quantify analytes at extremely low concentrations—often in the picogram per milliliter (pg/mL) range or lower—while maintaining high analytical selectivity.
Designed to detect analytes at pg/mL or lower, these strategies maintain selectivity in highly complex biological matrices. Critical for:
- Biologics and peptides at low doses
- Oligonucleotides with limited systemic exposure (LC-MS Bioanalysis for Oligonucleotides)
- ADC payload quantification
Selectivity is equally critical, as biological matrices contain thousands of endogenous components that can interfere with detection. Advanced strategies employ optimized sample preparation, chromatographic separation, and selective detection techniques to ensure accurate measurement of the target analyte without interference.
2.2 Ability to Handle Molecular Complexity
Modern therapeutics rarely exist as a single, uniform molecular entity. Instead, they often appear as heterogeneous populations comprising intact molecules, truncated forms, metabolites, and chemically or biologically modified variants.
Advanced Bioanalytical Strategies are specifically designed to:
- Differentiate between structurally related molecular species
- Quantify multiple forms of the same therapeutic when required
- Monitor biotransformation and degradation pathways
This capability is particularly important for biologics, ADCs, and nucleic acid–based therapies, where different molecular forms may have distinct pharmacological or safety implications.
2.3 Orthogonal Analytical Confirmation
A defining feature of Advanced Bioanalytical Strategies is the use of orthogonal analytical techniques to confirm data accuracy and reliability.
Ensures accuracy and reliability through multiple complementary methods:
- LC-MS/MS for quantitation (LC-MS/MS Bioanalytical Services)
- Ligand-binding assays (LBAs) for functional measurement (Biomarker Bioanalytical Services)
developers can validate results through independent measurement principles. Orthogonal confirmation reduces analytical bias, improves data confidence, and strengthens the scientific and regulatory defensibility of bioanalytical datasets.
2.4 Regulatory-Aligned Method Validation
Methods validated per FDA, EMA, ICH guidelines ensure reproducibility, sensitivity, and selectivity (Bioanalytical Method Development & Validation).
Regulatory-aligned validation ensures:
- Consistent accuracy and precision across studies
- Demonstrated selectivity and sensitivity in relevant matrices
- Verified stability under storage and processing conditions
- Reproducibility across analysts, instruments, and study phases
This alignment ensures that bioanalytical data are fit for regulatory submissions, inspections, and long-term clinical development.
2.5 Scalability Across Drug Development Phases
Another critical characteristic of Advanced Bioanalytical Strategies is their scalability across the drug development lifecycle.Methods support discovery, preclinical, clinical, and post-approval studies without loss of comparability (Bioanalytical Services in Drug Development).
Methods are designed to:
- Support early discovery and preclinical studies
- Transition smoothly into clinical development
- Remain relevant for late-stage trials and post-approval activities
This scalability minimizes method redevelopment, preserves data continuity, and ensures comparability across studies. As a result, early-phase bioanalytical data remain scientifically meaningful and regulatorily defensible throughout the lifecycle of the drug.
3: Why Complex Drug Modalities Require Advanced Bioanalytical Strategies
Complex drug modalities behave fundamentally differently from small molecules, making Advanced Bioanalytical Strategies essential rather than optional.
Unlike small molecules, complex therapeutics often exhibit:
- Structural diversity
- Biological instability
- Target-mediated disposition
- Multiple active or inactive molecular forms
Conventional methods cannot meet these analytical demands (Challenges in Bioanalytical Method Development).
4: Challenges Posed by Complex Drug Modalities
Complex drug modalities—including biologics, oligonucleotides, peptides, and antibody-drug conjugates (ADCs)—present unique analytical challenges that cannot be addressed by conventional small-molecule bioanalytical methods. These challenges arise from the inherent structural complexity, biological instability, and low systemic exposure of these therapeutics. Advanced Bioanalytical Strategies are specifically designed to overcome these hurdles, ensuring accurate, reliable, and regulatory-compliant data.
4.1 Structural Heterogeneity and Multiple Active Forms
Many modern therapeutics exist as heterogeneous populations rather than uniform molecules. For example:
- Biologics: glycoforms, isoforms, truncated variants
- ADCs: fully vs partially conjugated antibodies (Antibody-Drug Conjugate Bioanalytical Services)
- Oligonucleotides: metabolites of varying chain lengths
Each species may have distinct pharmacological or safety profiles, making it critical to differentiate and quantify each form accurately. Advanced bioanalytical strategies, including LC-MS/MS and orthogonal techniques, provide the resolution and selectivity necessary to measure these heterogeneous species reliably.
4.2 Enzymatic Degradation and Metabolic Instability
Peptides, oligonucleotides, and other biomolecules are inherently unstable in biological matrices due to enzymatic activity:
- Proteases can rapidly cleave peptides or proteins.
- Nucleases degrade RNA or DNA therapeutics.
- Mitigation with SPE, immunoaffinity capture, and optimized chromatography (Bioanalytical Matrix Effects)
Without advanced sample stabilization, rapid quenching, and optimized extraction techniques, analyte degradation can lead to underestimation of exposure, misleading PK/TK profiles, and compromised safety interpretation.
4.3 Matrix Interference from Endogenous Biomolecules
Biological matrices contain thousands of endogenous components that can interfere with analyte detection. Common sources of interference include:
- Proteins and lipoproteins
- Phospholipids and salts
- Endogenous nucleic acids or metabolites
Matrix effects can suppress ionization in mass spectrometry or generate false positives in immunoassays. Advanced cleanup strategies—such as solid-phase extraction (SPE), immunoaffinity capture, and optimized chromatographic separation—are essential to mitigate these effects and ensure accurate quantitation.
4.4 Extremely Low Circulating Concentrations
Many next-generation therapeutics are highly potent and administered at very low doses. For example:
- ADC payloads may circulate at sub-nanogram per milliliter levels.
- Requires ultra-sensitive LC-MS/MS with isotope-labeled standards (LC-MS/MS Bioanalysis of Xenobiotics)
Detecting these low concentrations requires ultra-sensitive analytical methods with high signal-to-noise ratios, such as LC-MS/MS with stable isotope-labeled internal standards.
4.5 Need for Both Quantitative and Qualitative Data
Complex modalities often demand more than just concentration measurements. Advanced bioanalytical strategies provide:
- Structural confirmation: Verifying the integrity and identity of the drug molecule.
- Metabolite profiling: Tracking biotransformation products that may affect safety or efficacy.
- Impurity characterization: Detecting low-level modifications, aggregation, or degradation products.
- Supports PK/PD modeling, safety evaluation, and regulatory submissions (Bioanalytical Services for IND/ANDA Submissions)
Integrating quantitative and qualitative data ensures comprehensive understanding of the drug’s pharmacokinetics, pharmacodynamics, and safety profile.
Without Advanced Bioanalytical Strategies, these challenges can compromise pharmacokinetic (PK), bioavailability, toxicokinetic (TK), and safety assessments.
5: Types of Complex Drug Modalities Driving Advanced Bioanalysis
5.1 Biologics and Monoclonal Antibodies
Biologics require Advanced Bioanalytical Strategies to distinguish between:
- Intact antibodies
- Truncated or degraded forms
- Post-translationally modified variants
High selectivity is essential to avoid interference from endogenous immunoglobulins.
5.2 Oligonucleotides and RNA-Based Therapeutics
Oligonucleotides present unique challenges due to:
- Chain length variability
- Sequence specificity
- Rapid metabolism
Advanced strategies often employ hybrid LC-MS/MS and ligand-binding approaches to accurately measure parent compounds and metabolites.
5.3 Antibody-Drug Conjugates (ADCs)
ADCs are among the most analytically complex modalities and require multi-analyte bioanalytical strategies to monitor:
- Total antibody (regardless of conjugation status)
- Conjugated drug (active ADC)
- Free cytotoxic payload
Each analyte provides distinct pharmacological and safety insights.
5.4 Peptides and Protein Therapeutics
Peptides degrade rapidly in biological matrices, requiring:
- Specialized stabilization
- Rapid sample processing
- Optimized extraction techniques
Advanced strategies ensure reliable exposure assessment despite instability.
6: Core Technologies Powering Advanced Bioanalytical Strategies
6.1 LC-MS/MS: The Backbone of Advanced Bioanalysis
Advanced Bioanalytical Strategies rely heavily on LC-MS/MS due to its unmatched analytical performance.
Why LC-MS/MS Is Critical
- Precise quantification at pg/mL levels
- Exceptional specificity in complex matrices
- Ability to simultaneously measure parent drugs and metabolites
- Broad applicability across drug modalities
LC-MS/MS forms the foundation of most modern advanced bioanalytical workflows.
6.2 High-Resolution Mass Spectrometry (HRMS)
HRMS plays a critical role in Advanced Bioanalytical Strategies, particularly during early development.
HRMS Supports:
- Structural confirmation of analytes
- Comprehensive metabolite identification
- Impurity and degradation product profiling
This capability is essential for mechanistic understanding and regulatory documentation.
6.3 Hybrid LBA–LC-MS Approaches
Hybrid assays combine the strengths of immunoassays and mass spectrometry.
Key Components:
- Immunocapture for high specificity
- Mass spectrometry for accurate quantitation
These approaches are especially effective for large molecules, biologics, and oligonucleotides where selectivity and sensitivity are both critical.
7: Method Development in Advanced Bioanalytical Strategies
The success of Advanced Bioanalytical Strategies begins with intelligent and scientifically grounded method development.
Key Considerations in Method Design
- Comprehensive understanding of molecular structure and behavior
- Selection of appropriate internal standards (often isotope-labeled)
- Optimization of sample extraction and cleanup
- Robust mitigation of matrix effects
At ResolveMass Laboratories Inc., every method is designed to be fit-for-purpose, ensuring reliability without unnecessary complexity.
8: Bioanalytical Method Validation for Complex Modalities
Advanced Bioanalytical Strategies must meet stringent global regulatory expectations.
Commonly Addressed Validation Parameters
| Parameter | Purpose |
|---|---|
| Accuracy & Precision | Ensure reproducibility and reliability |
| Selectivity | Prevent interference from endogenous components |
| Sensitivity | Enable low-dose and micro-dosing studies |
| Stability | Confirm analyte integrity during storage and processing |
| Recovery | Ensure consistent extraction efficiency |
Regulatory-ready validation supports smooth progression into clinical development.
9: Managing Matrix Effects and Interferences
Matrix effects are a major analytical challenge that Advanced Bioanalytical Strategies are specifically designed to overcome.
Common Mitigation Techniques
- Stable isotope-labeled internal standards
- Solid-phase extraction (SPE)
- Immunoaffinity purification
- Optimized chromatographic separation
These approaches significantly improve data accuracy, precision, and confidence.
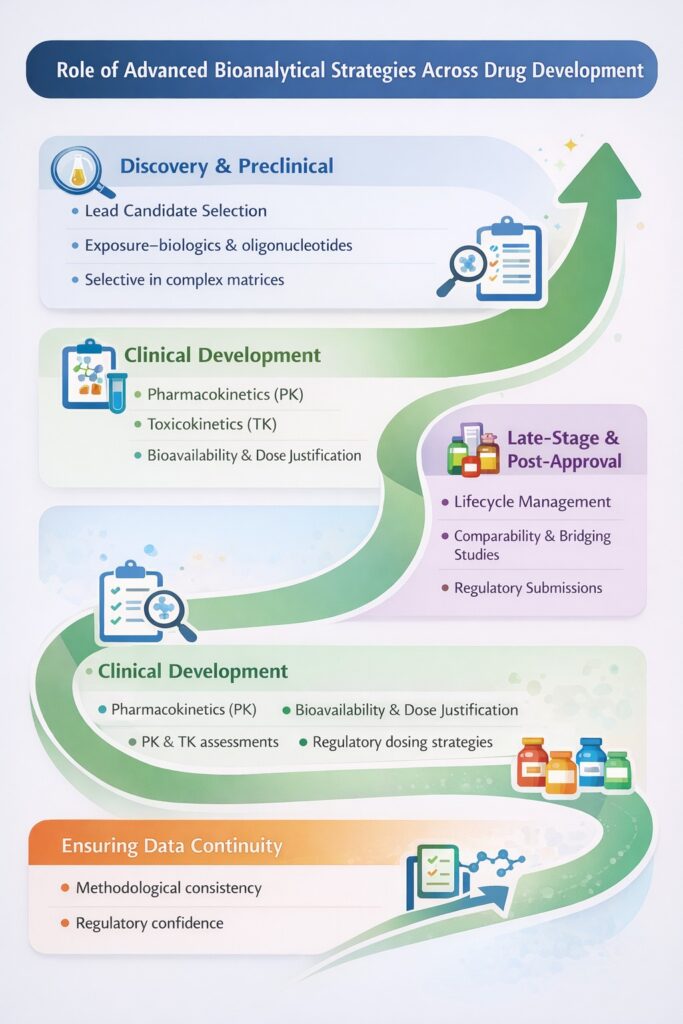
10: Role of Advanced Bioanalytical Strategies Across Drug Development
Advanced Bioanalytical Strategies play a critical role throughout the entire drug development lifecycle by generating high-quality, reliable, and interpretable data that directly support scientific decisions and regulatory submissions. From early discovery to post-approval lifecycle management, advanced bioanalysis ensures data continuity, methodological consistency, and regulatory confidence across all phases.
10.1 Discovery and Preclinical Phase
In the discovery and preclinical stages, Advanced Bioanalytical Strategies enable informed decision-making by providing early insights into exposure, behavior, and safety of drug candidates.
10.1.1 Lead Candidate Selection
Advanced bioanalysis supports the comparison of multiple candidates by delivering accurate and sensitive exposure data. These strategies help identify molecules with optimal pharmacokinetic profiles, stability, and bioavailability, enabling rational lead selection.
Key contributions include:
- Quantification of drug levels in plasma and tissues
- Assessment of metabolic stability and clearance
- Identification of early structure–exposure relationships
10.1.2 Exposure–Response Characterization
Understanding the relationship between drug exposure and biological effect is essential during preclinical development. Advanced bioanalytical strategies provide reliable concentration data that feed into pharmacology and efficacy models.
This allows:
- Establishment of dose–exposure–response relationships
- Optimization of dosing regimens
- Selection of starting doses for first-in-human studies
10.1.3 Early Safety and Toxicology Support
Preclinical safety assessment relies heavily on accurate toxicokinetic (TK) data. Advanced bioanalysis ensures precise measurement of systemic exposure during toxicology studies, even at low or fluctuating concentrations.
Key outcomes include:
- Identification of exposure margins
- Correlation of toxicity with systemic exposure
- Support for safety pharmacology and regulatory toxicology packages
10.2 Clinical Development
During clinical development, Advanced Bioanalytical Strategies become central to demonstrating safety, efficacy, and appropriate dosing in human subjects.
10.2.1 Pharmacokinetics (PK)
Advanced bioanalysis enables accurate determination of pharmacokinetic parameters such as Cmax, Tmax, AUC, clearance, and half-life.
These data support:
- Dose escalation and optimization
- Assessment of inter-subject variability
- Understanding of target-mediated drug disposition for complex modalities
10.2.2 Toxicokinetics (TK)
Advanced bioanalytical strategies ensure continuity between nonclinical and clinical exposure data. TK assessments help link observed adverse effects to systemic drug levels, strengthening safety interpretation.
This supports:
- Translation of preclinical safety findings to humans
- Risk assessment during dose escalation
- Regulatory justification of clinical dosing strategies
10.2.3 Bioavailability and Dose Justification
Accurate bioanalytical data are essential for evaluating bioavailability across formulations, routes of administration, or dosing regimens.
Advanced bioanalysis supports:
- Absolute and relative bioavailability studies
- Food-effect evaluations
- Dose justification in regulatory submissions
10.3 Late-Stage and Post-Approval Development
As products move toward commercialization and beyond, Advanced Bioanalytical Strategies ensure long-term data reliability and regulatory compliance.
10.3.1 Lifecycle Management
Advanced bioanalysis supports formulation changes, manufacturing scale-up, and process optimization without compromising data comparability.
Applications include:
- Evaluation of formulation modifications
- Support for new indications or populations
- Long-term exposure monitoring
10.3.2 Comparability and Bridging Studies
When manufacturing changes occur, regulatory agencies require demonstration of comparability. Advanced bioanalytical strategies enable sensitive and reliable comparison of pre- and post-change material.
Key contributions:
- Demonstration of analytical and clinical comparability
- Support for biosimilarity or process changes
- Bridging data between clinical studies
10.3.3 Regulatory Submissions and Post-Marketing Support
Regulatory submissions depend on bioanalytical data that are robust, traceable, and compliant with global guidelines.
Advanced bioanalysis ensures:
- Audit-ready datasets
- Consistent methodology across studies
- Support for post-marketing commitments and pharmacovigilance
10.4 Ensuring Data Continuity Across All Phases
A defining advantage of Advanced Bioanalytical Strategies is their ability to maintain methodological consistency and data integrity across the entire drug development lifecycle. By using scalable, validated, and regulatory-aligned approaches, developers can ensure that early-phase data remain relevant and defensible through late-stage development and post-approval activities.
11: Why Outsourcing Advanced Bioanalytical Strategies Makes Sense
Developing in-house advanced bioanalytical capabilities requires substantial investment in instrumentation, expertise, and regulatory systems.
Benefits of Partnering with ResolveMass Laboratories Inc.
- Deep expertise in complex drug modalities
- Regulatory-compliant analytical workflows
- Advanced LC-MS and HRMS platforms
- Faster turnaround times
- Scientifically robust and defensible data
This partnership approach accelerates development while maintaining data quality.
12: Ensuring Data Integrity and Trustworthiness
Data integrity is a fundamental pillar of Advanced Bioanalytical Strategies.
ResolveMass Laboratories Inc. ensures:
- Compliance with ALCOA+ principles
- Secure and traceable data systems
- Audit-ready documentation
- Transparent and reproducible reporting
This ensures confidence for both sponsors and regulatory agencies.
Future Trends in Advanced Bioanalytical Strategies
The field continues to evolve rapidly, driven by innovation and regulatory expectations.
Emerging Trends Include:
- Automation and high-throughput workflows (High-Throughput Bioanalysis)
- Micro- and nano-sampling techniques
- AI-assisted data processing (Bioanalytical CRO for Virtual Biotech)
- Multi-attribute methods (MAM) for biologics (LC-MS for Large Molecules)
These advancements will further strengthen Advanced Bioanalytical Strategies in supporting complex drug development.
Core Technologies, Method Development, Validation, Matrix Effects, and Role Across Drug Development
- Bioanalytical Method Development
- Bioanalytical Testing Services Cost
- Clinical Bioanalytical Services
- High-Throughput Bioanalysis
- Regulated Bioanalytical Services
- Bioanalytical Laboratory Services
- Cell and Gene Therapy Bioanalysis
- Bioanalytical CRO
Why Outsourcing Advanced Bioanalytical Strategies Makes Sense
- Bioanalytical CRO Services for PK and TK
- Bioanalytical Outsourcing CRO
- Affordable Bioanalytical Services for Biotech Startups
- Outsource Bioanalysis for Biotech Startups
- Virtual Bioanalytical Strategy
Conclusion
In an era dominated by complex drug modalities, Advanced Bioanalytical Strategies are no longer optional—they are foundational. From biologics and oligonucleotides to ADCs and gene therapies, advanced bioanalysis ensures accurate characterization, regulatory compliance, and confident decision-making.
ResolveMass Laboratories Inc. delivers deep scientific expertise, proven analytical methodologies, and regulatory awareness—helping sponsors navigate complexity with clarity and confidence through robust Advanced Bioanalytical Strategies.
Frequently Asked Questions:
Bioanalytical modalities refer to the types of therapeutic molecules that require bioanalysis. The major bioanalytical modalities include:
-Small molecules
-Biologics (monoclonal antibodies, proteins)
-Peptides
-Oligonucleotides and RNA-based therapeutics
-Antibody–drug conjugates (ADCs)
-Cell and gene therapies
Each modality requires different bioanalytical strategies due to differences in size, structure, and biological behavior.
Advanced analytical techniques are used to achieve high sensitivity, selectivity, and accuracy. Common techniques include:
-LC-MS/MS (Liquid Chromatography–Tandem Mass Spectrometry)
-HPLC and UPLC
-High-Resolution Mass Spectrometry (HRMS)
-GC-MS
-NMR spectroscopy
-Ligand-binding assays (ELISA)
-Hybrid LBA–LC-MS techniques
These techniques are essential for analyzing complex drug molecules and biological samples.
Advanced drug administration techniques are designed to improve targeting, efficacy, and patient compliance. Examples include:
-Targeted drug delivery systems
-Nanoparticle-based delivery
-Liposomes and micelles
-Controlled and sustained-release formulations
-Transdermal drug delivery systems
-Gene and cell-based delivery systems
These approaches help reduce side effects and improve therapeutic outcomes.
Drug targeting strategies aim to deliver drugs specifically to the desired site of action. Key strategies include:
-Passive targeting (e.g., enhanced permeability and retention effect)
-Active targeting (ligand–receptor interactions)
-Physical targeting (using heat, magnetic fields, or light)
-Prodrug-based targeting
-Antibody-mediated targeting
These strategies improve efficacy and safety by minimizing off-target effects.
In the pharmaceutical industry, it is used for:
-Deviations and OOS investigations
-CAPA (Corrective and Preventive Action)
-Quality failures
-Process improvements
The goal is to identify the root cause, not just treat symptoms.
The two main types of pharmaceutical analysis are:
-Qualitative analysis – Identifies the presence or identity of a drug or impurity
-Quantitative analysis – Measures the amount or concentration of a drug or impurity
Both are essential for quality control and regulatory compliance.
The four main types of validation in the pharmaceutical industry are:
-Process validation – Ensures manufacturing processes produce consistent quality
-Analytical method validation – Confirms accuracy, precision, and reliability of test methods
-Cleaning validation – Ensures equipment is free from residues and contaminants
-Computer system validation (CSV) – Ensures computerized systems perform as intended
These validations ensure product quality, safety, and regulatory compliance.
Reference
- Bioanalysis in the Age of New Drug Modalities.https://link.springer.com/article/10.1208/s12248-021-00594-w
- Bioanalytical Methods and Strategic Perspectives Addressing the Rising Complexity of Novel Bioconjugates and Delivery Routes for Biotherapeutics.https://pmc.ncbi.nlm.nih.gov/articles/PMC8972746/
- Aarzoo Thakur, Zhiyuan Tan,T subasa Kameyama, Eman El-Khateeb, Shakti Nagpal, Stephanie Malone.Bioanalytical strategies in drug discovery and development.https://www.tandfonline.com/doi/abs/10.1080/03602532.2021.1959606
- Chapter 1 – Bioanalysis: methods, techniques, and applications.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780128226544000026

