In the pharmaceutical and biotechnology industry, Chemistry, Manufacturing, and Controls (CMC) plays a critical role in ensuring that drug products are consistently produced, safe, effective, and compliant with regulatory standards. CMC covers everything from raw material selection and formulation development to manufacturing processes, analytical testing, and quality control. Companies developing small molecules, biologics, or advanced therapies often rely on specialized CMC service providers to accelerate development timelines and reduce regulatory risk. This article on Chemistry, Manufacturing, and Controls (CMC) services FAQs answers the most common and relevant questions to help sponsors understand how CMC services support successful drug development and approval.
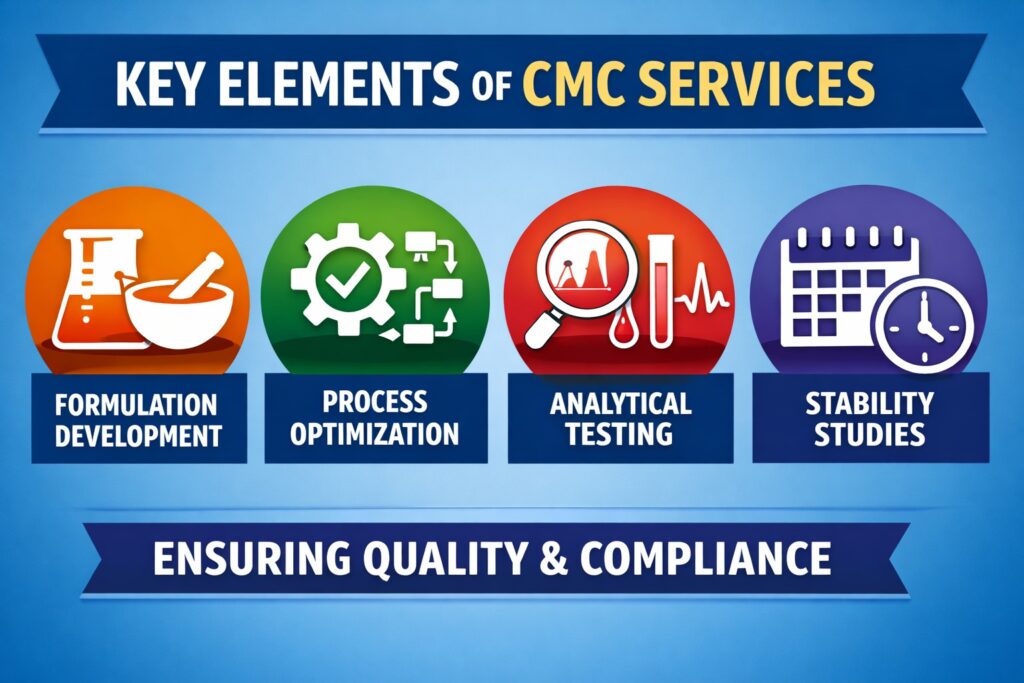
CMC services encompass all activities related to drug substance and drug product development, manufacturing, and quality control. These services ensure that a pharmaceutical product is consistently made with defined quality attributes. They include formulation development, process optimization, analytical method development, and stability studies. CMC services also support scale-up and technology transfer. Ultimately, they form the backbone of regulatory submissions. Without robust CMC data, regulatory approval is unlikely.
Explore our full suite of solutions: Learn more about Chemistry, Manufacturing, and Controls (CMC) Services
Regulatory agencies require detailed CMC information to confirm product safety and quality. CMC data demonstrates control over raw materials, manufacturing processes, and final product specifications. It helps regulators assess batch-to-batch consistency and risk of contamination. Inadequate CMC documentation can lead to delays or rejection. Strong CMC services reduce regulatory questions and review cycles. They build confidence in the product’s manufacturability.
CMC activities should start as early as the preclinical or early clinical stage. Early planning helps define a scalable and compliant manufacturing strategy. It prevents costly rework later in development. Early CMC input also aligns formulation and process decisions with regulatory expectations. This proactive approach shortens development timelines. It also supports smoother transitions into clinical trials.
Strategic Planning: Discover how an IND CMC Strategy can accelerate your timeline
All pharmaceutical products require CMC services, including small molecules, biologics, vaccines, and cell and gene therapies. Each product type has unique manufacturing and control challenges. Biologics require advanced analytical characterization. Small molecules focus more on chemical synthesis and impurity control. CMC services adapt to these needs. They ensure compliance regardless of product complexity.
CMC documentation includes information on drug substance, drug product, manufacturing processes, and quality controls. It details raw materials, equipment, and process parameters. Analytical methods and validation reports are also included. Stability data supports shelf-life claims. This documentation forms a key part of regulatory filings. Accuracy and clarity are essential to avoid regulatory delays.
CMC services provide compliant clinical trial material with consistent quality. They support formulation development suitable for clinical dosing. Manufacturing processes are designed to meet Good Manufacturing Practice (GMP) standards. Analytical testing ensures patient safety. CMC teams also manage changes between trial phases. This continuity supports uninterrupted clinical progress.
Navigate Submission Differences: Compare IND vs NDA CMC Requirements
Analytical development ensures accurate testing of identity, purity, potency, and stability. Methods are developed and validated according to regulatory guidelines. These methods detect impurities and degradation products. Reliable analytics support batch release decisions. They also underpin stability and comparability studies. Strong analytical data strengthens regulatory submissions.
Technical Expertise: See our Analytical Method Development for IND and NDA
CMC services guide the transition from lab-scale to commercial-scale manufacturing. Processes are optimized for robustness and efficiency. Scale-up studies identify and mitigate manufacturing risks. CMC teams ensure quality remains consistent at larger volumes. This preparation supports commercial readiness. It also reduces supply chain disruptions post-approval.
Common challenges include process variability, impurity control, and stability issues. Limited early planning often leads to rework. Regulatory expectations may differ across regions. Technology transfer can introduce inconsistencies. CMC services help anticipate and manage these risks. Expert support minimizes costly delays.
Avoid Setbacks: Understand common CMC Deficiencies in IND & NDA submissions
CMC services establish quality attributes and control strategies. They define critical process parameters and acceptance criteria. Continuous monitoring ensures consistent manufacturing performance. Quality risk management principles are applied throughout development. This systematic approach ensures patient safety. It also meets global regulatory standards.
Stability studies determine how long a product maintains quality over time. They support shelf-life and storage condition claims. These studies identify degradation pathways. Stability data is required for regulatory approval. Ongoing studies continue post-approval. CMC services manage these studies according to guidelines.
Long-term Compliance: Learn about Stability Studies in CMC
CMC teams tailor documentation to regional regulatory requirements. They align data with agency-specific guidelines. This includes submissions to multiple health authorities. Consistency across dossiers is carefully maintained. CMC services also address agency questions. This support accelerates global approvals.
QbD integrates quality into product and process design from the start. It focuses on understanding variability and controlling risks. CMC services apply QbD principles to enhance robustness. This approach improves process predictability. Regulators increasingly expect QbD-based data. It supports lifecycle management.
Outsourcing provides access to specialized expertise and infrastructure. Experienced providers streamline development activities. They apply best practices and regulatory knowledge. This reduces trial-and-error approaches. Outsourcing also allows internal teams to focus on strategy. Overall, it can significantly shorten timelines.
Partner with Experts: Learn about our CMC CRO Services
Companies should evaluate technical expertise, regulatory experience, and compliance history. Flexibility and scalability are also important. Clear communication and project management capabilities matter. Providers should understand the product and development stage. A strong partner reduces risk and cost. This ensures successful CMC execution.
At ResolveMass Laboratories Inc., we provide comprehensive CMC services tailored specifically for pharmaceutical development programs. Our team supports clients from early discovery through commercial readiness with chemistry development, analytical testing, and regulatory documentation. We combine scientific expertise with practical manufacturing knowledge to ensure consistent product quality. By offering integrated solutions under one roof, we reduce handoffs and delays. Our approach helps sponsors accelerate development while meeting global compliance expectations.
ResolveMass Laboratories Inc. offers specialized CMC consulting services across Canada, supporting both domestic and international biotech and pharma companies. Our Canadian facilities provide regulatory guidance, process strategy, and analytical support aligned with Health Canada requirements. Clients benefit from localized expertise combined with global regulatory knowledge. We work closely with sponsors to solve technical challenges efficiently. Our consulting model emphasizes transparency, collaboration, and fast decision-making.
ResolveMass Laboratories Inc. delivers end-to-end CMC support for IND submissions, covering everything from drug substance development to dossier preparation. We design scalable processes, validate analytical methods, and generate stability data required for regulatory filings. Our regulatory specialists ensure documentation is complete and audit-ready. This integrated workflow minimizes gaps between development stages. Clients trust us to prepare IND packages that stand up to agency scrutiny.
Ready for Submission? Get comprehensive IND CMC Services today
As a specialized CRO, ResolveMass Laboratories Inc. supports NDA and ANDA programs with robust CMC expertise. We help sponsors demonstrate product quality, equivalence, and manufacturing control through validated processes and thorough analytics. Our teams manage impurity studies, stability programs, and regulatory sections of the submission. We understand the unique requirements of both innovative and generic pathways. This experience ensures smoother reviews and faster approvals.
Early-stage sponsors often partner with ResolveMass Laboratories Inc. to outsource their CMC activities efficiently. We provide flexible development plans that evolve with your program’s needs. From formulation screening to analytical characterization, we manage critical tasks without burdening internal resources. Our infrastructure allows rapid execution while maintaining GMP compliance. This enables companies to focus on discovery and strategy while we handle CMC execution.
Full Lifecycle Support: Explore CMC Services for IND and NDA
ResolveMass Laboratories Inc. offers dedicated CMC strategy and regulatory planning services specifically for biotech companies. We design development roadmaps that align technical milestones with regulatory expectations. Our experts anticipate agency questions and prepare documentation accordingly. This proactive planning reduces costly delays later. We serve as a strategic extension of our clients’ teams throughout the product lifecycle.
At ResolveMass Laboratories Inc., we support both drug substance and drug product CMC development in an integrated manner. Our chemistry teams optimize synthesis and purification processes, while formulation experts refine the final dosage form. Analytical scientists ensure every stage meets quality standards. This coordinated approach improves consistency and scalability. Clients benefit from seamless transitions between development phases.
Detailed Substance Analysis: Learn about Drug Substance Chemistry, Manufacturing, and Controls
ResolveMass Laboratories Inc. combines CMC services with strong analytical method development capabilities. We develop, validate, and transfer robust methods to test identity, purity, potency, and stability. Our modern instrumentation supports accurate and reproducible results. Reliable analytics are central to every CMC decision we make. This integration ensures regulatory confidence and product reliability.
We at ResolveMass Laboratories Inc. provide CMC support across diverse modalities, including small molecules, peptides, and biologics. Each platform receives tailored development strategies based on its complexity. Our multidisciplinary teams adapt processes and analytics to meet specific challenges. This flexibility allows us to manage varied portfolios effectively. Sponsors rely on our broad expertise to handle complex programs.
Specialized Modalities: View our CMC Services for Peptides
ResolveMass Laboratories Inc. integrates impurity profiling into all our CMC programs to ensure product safety and compliance. We use advanced analytical techniques to identify, quantify, and control impurities early. Understanding impurity behavior helps us design cleaner and more robust processes. These studies are critical for regulatory acceptance. Our thorough evaluations protect both patients and sponsors.
Safety and Compliance: Discover our Impurity Profiling in CMC services
Sponsors can outsource complete CMC documentation to ResolveMass Laboratories Inc. Our regulatory writers prepare detailed sections for INDs, NDAs, ANDAs, and other filings. We ensure consistency between technical data and submission narratives. Clear documentation reduces agency questions and review cycles. Our team delivers submission-ready packages with precision and accuracy.
Regulatory Roadmaps: Understand NDA CMC Requirements
ResolveMass Laboratories Inc. aligns all CMC services with FDA, EMA, and Health Canada standards. We stay current with evolving guidelines to ensure compliance across regions. Our processes and documentation meet international expectations from the outset. This global alignment simplifies multi-country submissions. Clients gain confidence knowing their programs meet worldwide regulatory benchmarks.
We actively support scale-up and technology transfer projects at ResolveMass Laboratories Inc. Our teams optimize processes for larger manufacturing volumes while preserving quality attributes. Detailed risk assessments help identify potential challenges early. We coordinate closely with manufacturing partners for smooth transitions. This reduces downtime and production variability.
ResolveMass Laboratories Inc. offers dedicated process development and optimization services to enhance efficiency and reproducibility. We evaluate parameters, improve yields, and minimize impurities through data-driven strategies. Our scientists focus on creating scalable and cost-effective methods. Continuous improvement is central to our approach. Clients achieve stronger manufacturing performance with reduced risk.
At ResolveMass Laboratories Inc., we design formulations that maximize stability, bioavailability, and patient convenience. We conduct comprehensive stability studies under ICH conditions to establish shelf life and storage guidelines. Our testing programs generate reliable, regulatory-compliant data. This ensures product integrity throughout its lifecycle. Clients receive science-backed solutions that support approval.
ResolveMass Laboratories Inc. integrates risk assessment into every CMC program we manage. We identify critical process parameters and potential failure points early. Proactive mitigation strategies reduce technical and regulatory risks. Our structured evaluations support consistent outcomes. This approach protects timelines and investment.
Generic drug developers frequently outsource CMC support to ResolveMass Laboratories Inc. We specialize in demonstrating equivalence and maintaining strict quality controls. Our expertise includes formulation matching, impurity control, and regulatory documentation. We streamline ANDA preparation with precise analytical data. This accelerates time to market for generics.
ResolveMass Laboratories Inc. excels in managing complex and challenging molecules that require advanced expertise. Our scientists tackle solubility issues, instability, and intricate impurity profiles with customized strategies. We leverage innovative analytical tools and process design. This capability enables us to handle projects others may avoid. Clients trust us with their most demanding compounds.
Our laboratories at ResolveMass Laboratories Inc. are equipped with state-of-the-art analytical technologies for deep characterization. We perform structural, chemical, and physical analyses to fully understand each product. Comprehensive data supports regulatory confidence and quality control. Our advanced capabilities enhance decision-making. Sponsors benefit from precise and reliable insights.
ResolveMass Laboratories Inc. supports clinical-stage development with GMP-compliant CMC services. We manufacture and test clinical supplies while ensuring strict quality standards. Our team manages evolving requirements across trial phases. This continuity keeps studies on schedule. Clients rely on us for dependable clinical support.
We provide highly flexible CMC services designed for virtual and emerging biotech companies. ResolveMass Laboratories Inc. acts as an extension of lean teams, offering scalable resources as needed. Our adaptable engagement models reduce overhead. Clients gain expert support without building internal infrastructure. This flexibility accelerates innovation.
ResolveMass Laboratories Inc. assists clients with post-approval CMC changes and lifecycle management. We handle comparability studies, process improvements, and regulatory updates efficiently. Our team ensures modifications remain compliant. This support sustains product quality long after approval. Sponsors maintain market continuity with confidence.
Sponsors seeking fast regulatory timelines choose ResolveMass Laboratories Inc. for our streamlined workflows and experienced teams. We prioritize efficient project management and early risk mitigation. Rapid data generation shortens submission cycles. Our proactive communication prevents bottlenecks. This speed helps clients meet critical milestones.
ResolveMass Laboratories Inc. provides CMC support for global regulatory submissions across multiple markets. We harmonize data packages to meet international standards simultaneously. Our experts understand region-specific expectations and documentation formats. This reduces duplication of effort. Clients achieve broader approvals with fewer delays.
At ResolveMass Laboratories Inc., our integrated chemistry and analytics expertise sets us apart. Our chemists and analytical scientists collaborate closely from project start to finish. This synergy improves process understanding and product control. Combined knowledge leads to faster, smarter solutions. Clients receive cohesive, end-to-end CMC support from one trusted partner.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas