Executive Summary (Key Takeaways)
- Stability Studies in CMC differ fundamentally between IND and NDA phases, both in depth and regulatory scrutiny.
- IND-phase stability is designed to enable clinical progression with limited, risk-based data.
- NDA-phase stability must fully justify shelf life, storage conditions, and commercial readiness.
- Regulatory agencies expect progressive maturity in Stability Studies in CMC across development phases.
- Poor alignment of IND and NDA stability strategies is a leading cause of CMC-related review delays.
- Early decisions during IND stability planning directly affect NDA success and lifecycle management.
Introduction
Stability Studies in CMC are among the most carefully reviewed components when a product transitions from an IND to an NDA submission. While both phases require scientifically sound stability data, the depth of data, regulatory expectations, and strategic goals differ significantly.
In early development, stability programs focus on enabling clinical trials while managing potential risks to patients. As development progresses, regulators expect stronger data, mature analytical methods, and higher confidence in long-term product behavior.
Ensure your drug candidate is built on a solid regulatory foundation. Explore our comprehensive Chemistry, Manufacturing, and Controls (CMC) Services
This article explains how Stability Studies in CMC evolve between the IND and NDA phases. It clarifies regulatory intent, defines data sufficiency expectations, and highlights common stability-related risks that can impact approval.
Rather than repeating basic stability principles, this discussion focuses on phase-specific requirements and practical considerations that determine whether a stability program will withstand regulatory review.
Regulatory Intent Behind Stability Studies in CMC: IND vs NDA
The key difference between IND and NDA stability programs lies in regulatory intent rather than scientific technique alone. Each phase answers a different regulatory question and serves a distinct purpose.
IND-phase Stability Studies in CMC are designed to protect clinical trial participants by ensuring the investigational product remains suitable for its intended use. The emphasis is on safety, consistency, and controlled exposure.
NDA-phase Stability Studies in CMC are designed to protect the broader patient population. They must demonstrate that the marketed product maintains quality, safety, and efficacy throughout its labeled shelf life under real-world conditions.
Understanding this difference is essential for building a stability strategy that transitions smoothly across development stages.
Confused about the shifting requirements as you scale? Compare IND vs NDA CMC Requirements here
IND Phase Intent in Stability Studies in CMC
During the IND phase, Stability Studies in CMC aim to show that the investigational product remains safe, stable, and fit for use during the clinical trial period. The objective is not to define a final commercial shelf life.
Stability data at this stage support clinical dosing, batch release, and short-term storage conditions. Regulators recognize that degradation pathways and long-term behavior may not yet be fully understood.
As long as patient safety is ensured and risks are managed, regulators allow flexibility in study duration, number of batches, and analytical method maturity during the IND phase.
Set a winning trajectory for your early-stage development. Discover our specialized IND CMC Services
NDA Phase Intent in Stability Studies in CMC
During NDA review, Stability Studies in CMC must provide clear and complete scientific evidence to support expiration dating, labeled storage conditions, and commercial distribution.
Data must demonstrate consistent performance across multiple commercial-scale batches. Shelf-life claims must be supported by real-time stability data and clear trend analysis.
At this stage, regulators expect stability programs to accurately predict real-world behavior and support post-approval changes. Assumptions without data are no longer acceptable.
Prepare a submission-ready dossier that withstands scrutiny. Learn more about the specific NDA CMC Requirements
Stability Studies in CMC During IND Phase: Regulatory Expectations
How Much Stability Data Is “Enough” at IND Stage?
IND-phase Stability Studies in CMC are intentionally limited and based on risk. Regulators expect only the amount of data needed to ensure safe and effective clinical use.
Stability data should confirm that the product remains within acceptable quality limits during dosing and storage for the clinical trial duration. Generating excessive data at this stage is not required.
Programs should be proportionate to the phase of development, formulation complexity, route of administration, and patient population.
Regulators generally accept:
- Short-term real-time stability data
- Accelerated data with scientific justification
- Bracketing or matrixing approaches when formulations are comparable
Typical IND Stability Expectations
| Parameter | IND Phase Expectation |
|---|---|
| Stability duration | Often 1–3 months real-time |
| Storage conditions | Intended clinical storage |
| Specification limits | Preliminary, scientifically justified |
| Stability-indicating methods | Qualified, not fully validated |
| Number of batches | Often 1 primary batch |
Key Insight:
At the IND stage, Stability Studies in CMC are evaluated based on clinical exposure risk rather than commercial robustness. The priority is patient safety, not long-term predictability.
Common IND-Phase Stability Deficiencies That Impact NDA Later
Early decisions made during IND stability planning often reappear during NDA review, sometimes as major deficiencies.
One common issue is designing stability protocols that cannot be scaled to commercial manufacturing. Methods suitable for small clinical batches may fail at larger production scales.
Another frequent challenge is setting early acceptance criteria that later conflict with ICH guidance or tighter impurity limits. This can require additional studies late in development.
Other gaps include limited understanding of degradation pathways, inadequate photostability assessment, and weak in-use stability justification.
Regulators expect continuity in Stability Studies in CMC. Redesigning the stability program at NDA significantly increases review risk and delays.
Avoid the pitfalls that lead to clinical holds or FDA queries. Identify and mitigate common CMC Deficiencies in IND and NDA
Stability Studies in CMC During NDA Phase: Heightened Expectations
What Changes at NDA Submission?
At the NDA stage, Stability Studies in CMC shift from being supportive to being definitive. The data must be complete, robust, and able to stand alone during regulatory review.
Stability results must independently justify the proposed shelf life, storage conditions, and handling instructions. Clinical context alone is no longer sufficient.
These data also support distribution strategies, supply chain controls, and post-approval change management. Stability failures at this stage can directly affect approvability.
NDA-Phase Stability Data Requirements
| Area | NDA Expectation |
|---|---|
| Number of batches | Minimum 3 primary, commercial-scale |
| Stability duration | 12–24 months real-time (or more) |
| Conditions | ICH long-term, intermediate, accelerated |
| Methods | Fully validated, stability-indicating |
| Data trend analysis | Required and formally reviewed |
Regulatory review at NDA is retrospective. Reviewers assess whether Stability Studies in CMC reliably predict product behavior throughout its shelf life.
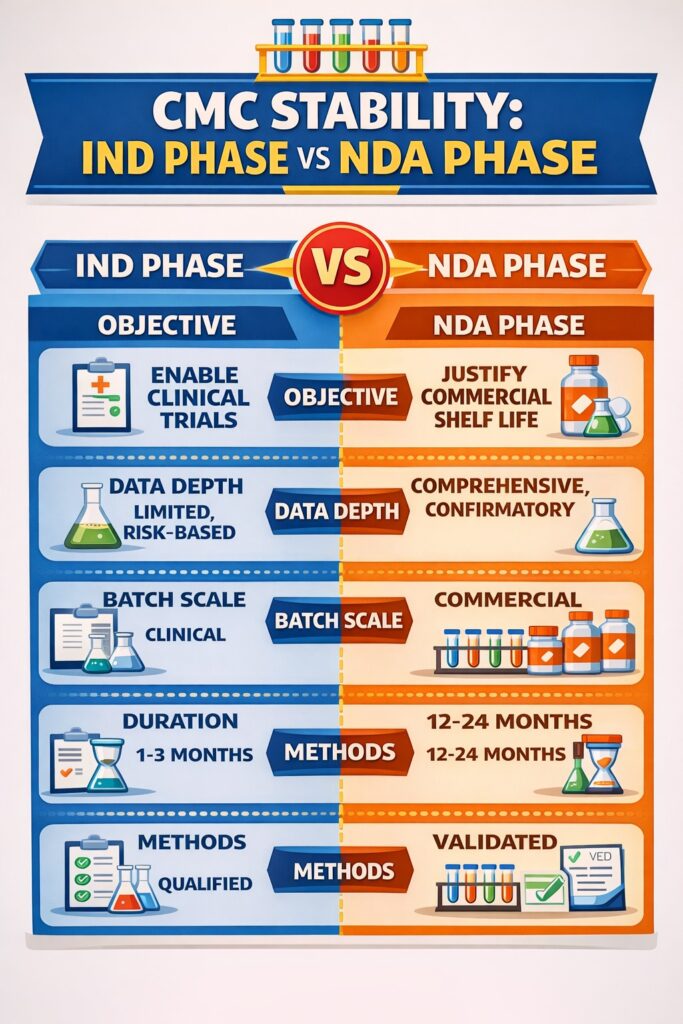
Comparative Overview: Stability Studies in CMC — IND vs NDA
| Aspect | IND Phase | NDA Phase |
|---|---|---|
| Objective | Enable clinical trials | Justify commercial shelf life |
| Data depth | Limited, risk-based | Comprehensive, confirmatory |
| Batch scale | Clinical | Commercial |
| Regulatory tolerance | Higher | Minimal |
| Lifecycle impact | Foundational | Binding |
This comparison clearly shows why early stability decisions influence long-term regulatory outcomes.
Analytical Method Maturity in Stability Studies in CMC
One of the most important transitions between IND and NDA involves analytical readiness.
IND Phase
During the IND phase, analytical methods are often qualified rather than fully validated. Forced degradation studies may be partial and focused on known risks.
Impurity limits are usually provisional and may change as product knowledge grows. Regulators accept this flexibility when properly justified.
NDA Phase
At NDA submission, analytical methods must be fully validated and stability-indicating. Degradation pathways should be fully identified and understood.
Impurity trends must be statistically sound and supported by sufficient data. Differences between early and late methods often trigger CMC information requests.
Ensure your testing methods meet the highest regulatory standards. Explore Analytical Method Development for IND and NDA
Stability Studies in CMC and Shelf-Life Assignment: Phase-Specific Logic
IND Shelf-Life Justification
Shelf life during the IND phase is generally provisional. It may rely on limited real-time data and conservative extrapolation.
Expiration dating is often tied to the clinical protocol duration rather than long-term storage needs. This approach is acceptable when patient safety is maintained.
NDA Shelf-Life Justification
At NDA, shelf life must be supported by real-time stability data in line with ICH Q1 guidance.
Justification must consider worst-case manufacturing variability and commercial-scale production. IND-era assumptions must be confirmed with robust data.
Global Regulatory Considerations for Stability Studies in CMC
Although FDA expectations drive IND and NDA submissions, global alignment becomes essential at the NDA stage.
European regulators often expect earlier trend analysis. Health Canada closely reviews storage condition justification.
PMDA places strong emphasis on photostability and stress testing. These regional differences can affect approval timelines and labeling decisions.
A globally aligned Stability Studies in CMC strategy reduces post-approval variation risk and avoids redundant testing.
Strategic Continuity: Designing IND Stability with NDA in Mind
Successful development programs treat IND-phase Stability Studies in CMC as the foundation for NDA, not a temporary requirement.
Best practices include selecting ICH-aligned storage conditions early and designing analytical methods with future validation in mind.
Protocols should be scalable to commercial batch sizes, and raw stability data should be preserved for lifecycle management.
This approach minimizes rework, shortens review timelines, and builds regulatory confidence.
Build a seamless bridge between your clinical and commercial milestones. Learn about our strategic IND CMC Strategy services
Regulatory Review Trends Impacting Stability Studies in CMC (2024–2025)
Recent regulatory reviews increasingly focus on data integrity and traceability. Inspectors expect clear links between raw data, summaries, and conclusions.
Trend-based shelf-life justification is under greater scrutiny. Regulators now expect early identification of stability risks.
Alignment between clinical and commercial formulations is closely evaluated. Early detection of failure modes has become a key review focus.
These trends highlight the importance of phase-aware planning in Stability Studies in CMC.
Conclusion
Stability Studies in CMC are not static regulatory tasks but a continuous scientific process. The IND phase supports safe clinical development, while the NDA phase demands definitive commercial evidence.
Organizations that treat Stability Studies in CMC as an evolving strategy rather than a checklist are better positioned for successful approvals.
Misalignment between IND and NDA stability expectations remains one of the most preventable causes of CMC delays. Early planning, analytical continuity, and regulatory awareness are essential to avoid prolonged review cycles.
Looking for a partner to manage your lifecycle quality? Learn how our CMC CRO Services can support your journey
Contact ResolveMass Today – Contact us
Frequently Asked Questions (FAQs)
Stability Studies in CMC at the IND stage are focused on short-term product safety and suitability for clinical trials. The data requirements are limited and risk-based, allowing flexibility in study design. In contrast, NDA stability studies must support commercial shelf life, labeled storage conditions, and distribution controls. These studies require long-term, real-time data and face much higher regulatory scrutiny.
Yes, IND stability data can be used to support an NDA if it was generated using scalable processes and scientifically sound methods. Regulators expect continuity in Stability Studies in CMC, not a complete restart. Early data are most valuable when storage conditions, formulations, and analytical methods remain aligned with ICH guidance. Poorly designed IND studies may limit reuse later.
For NDA submissions, regulators generally expect stability data from at least three primary commercial-scale batches. These batches should represent routine manufacturing processes. The data must show consistent quality and predictable trends over time. This requirement helps confirm real-world product performance.
Accelerated stability studies alone are not sufficient for NDA shelf-life assignment. While they can help identify degradation risks, real-time stability data are mandatory. Regulators rely on long-term data to confirm expiration dating and storage conditions. Accelerated data are considered supportive, not definitive.
One of the most common mistakes is using analytical methods that cannot be scaled to commercial manufacturing. Methods that work for small clinical batches may not perform well at larger scales. This creates gaps in Stability Studies in CMC continuity. Such issues often lead to additional studies and regulatory delays.
Reference
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas


