🔍 Summary – Key Takeaways
- Resolved complex FDA CMC queries within critical NDA review timelines.
- Strategic use of modular responses, risk-based justifications, and data bridging.
- Emphasis on analytical method validations, stability data corrections, and control strategy enhancement.
- Leveraged multidisciplinary SME teams with prior FDA-facing experience.
- Achieved NDA approval without major delay or complete response letter (CRL).
- Included post-resolution communication strategies to maintain FDA confidence.
- Optimized using real-world evidence (RWE) to support claims and eliminate re-queries.
- Offers a model approach for resolving FDA CMC queries efficiently.
Introduction: The Importance of Resolving FDA CMC Queries
During the NDA review process, resolving FDA CMC queries is critical for securing timely drug approvals. These queries often focus on Chemistry, Manufacturing, and Controls (CMC) details that the FDA reviews carefully. Even well-prepared submissions may face technical questions that must be answered quickly and precisely.
This case highlights how the sponsor overcame multiple CMC-related challenges, including method validation issues and stability data gaps. Success was achieved by combining scientific integrity with clear, timely communication. This experience reinforces the importance of having a reliable, cross-functional team and a well-defined regulatory strategy.
Learn how our experts support your regulatory journey: Explore our IND & CMC Case Studies for Drug Discovery
What Are FDA CMC Queries?
FDA CMC queries help confirm that a drug product can be safely and consistently manufactured. These questions usually target incomplete data, unclear justifications, or areas where risk controls need improvement. Addressing them requires both technical knowledge and a strong understanding of FDA expectations.
In this case, the sponsor received an Information Request (IR) and a Major Deficiency letter from the FDA during the NDA review. These types of queries indicated serious concerns that required urgent and detailed responses to avoid delays.
Partner with a team that understands the regulatory landscape: Discover our Comprehensive Drug Discovery CRO Chemistry Services
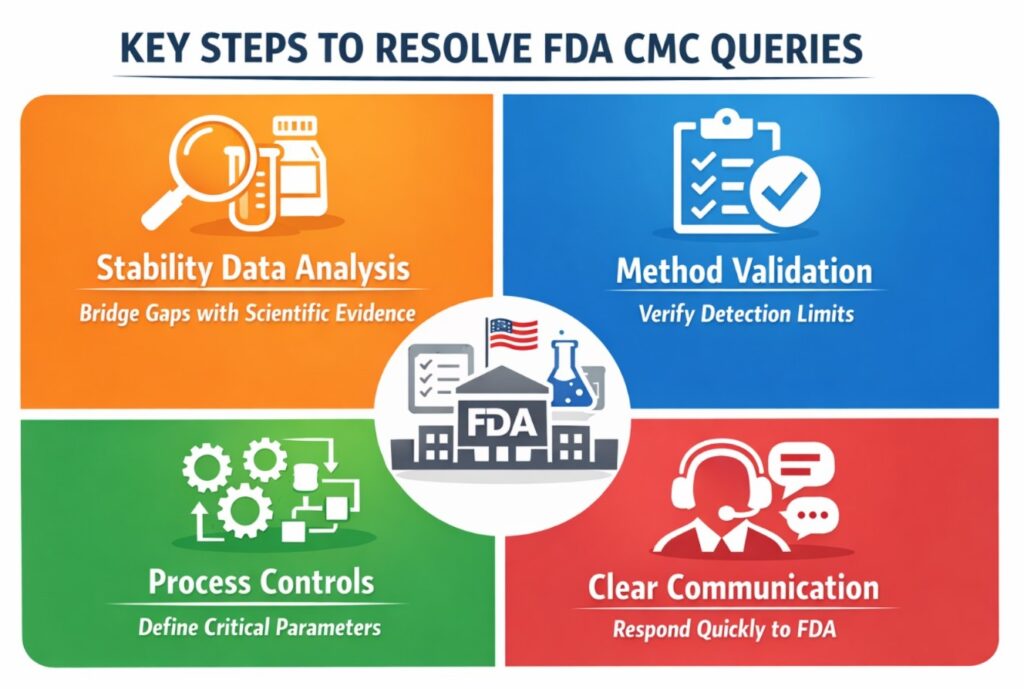
Key FDA CMC Queries Raised
| CMC Query Type | Description |
|---|---|
| Stability Studies | Incomplete long-term stability data for one dosage strength |
| Method Validation | Impurity detection limit not verified below ICH threshold |
| Process Controls | No defined Critical Process Parameters (CPPs) |
| Specification Justification | Unclear rationale for residual solvent limits |
These issues could have delayed approval if not handled thoroughly and in line with regulatory expectations.
A Structured Approach to Resolving FDA CMC Queries
The sponsor followed a clear framework for resolving FDA CMC queries, focusing on speed, accuracy, and regulatory alignment. Responses were backed by scientific data and written in a way that directly addressed the FDA’s concerns. The strategy also ensured that all parts of the NDA remained consistent and easy for reviewers to follow.
Access high-quality chemistry support for your NDA submission: Learn about our Custom Synthesis for Drug Discovery
Cross-Functional CMC War Room Setup
As soon as the FDA queries were received, a special CMC war room was created. This team included experts in Regulatory Affairs, Quality Assurance, Manufacturing, and Analytical Development. All had previous experience dealing with the FDA.
Weekly mock review sessions were held to test responses under real-world conditions. Subject Matter Experts (SMEs) ensured that technical accuracy and regulatory clarity were maintained. A single regulatory liaison managed all FDA communications to avoid confusion.
Scale your team with a dedicated external chemistry department: Explore our Virtual Chemistry Department CRO Services
Prioritizing Responses Based on Risk
The sponsor classified all FDA queries into high, medium, and low risk categories. This helped the team focus first on the most urgent issues that could have led to a CRL.
- High Risk: Gaps in stability data and lack of control strategies
- Medium Risk: Questions about method validation
- Low Risk: Minor edits or documentation updates
This prioritization helped reduce the risk of delays by focusing attention where it was most needed.
Specific Strategies for Resolving Key CMC Issues
Bridging Stability Data with Strong Scientific Support
To answer questions about stability data, the sponsor used accelerated stability studies along with real-time data. Statistical methods based on ICH guidelines (specifically ICH Q1E) were used to show consistency across batches and storage conditions.
A matrix-based approach was used to justify product shelf-life without needing to start new long-term studies. This method helped in resolving FDA CMC queries related to stability without extending the review timeline.
Identify and synthesize critical drug metabolites for your studies: View our Metabolite Synthesis Services for Drug Discovery
Enhancing Analytical Method Validation
The validation process for detecting impurities was updated to meet FDA expectations. New protocols were developed to show the method could detect impurities below the revised thresholds.
Supporting studies included spiked sample testing, robustness checks in multiple labs, and forced degradation studies. LC-MS analysis confirmed the method was specific and capable of detecting stability-related changes.
Ensure your methods meet the highest regulatory standards: Custom Analytical Method Development for Drug Discovery
Rebuilding the Control Strategy
The control strategy was revised in response to FDA concerns. New definitions were created for Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) based on risk assessments.
| Element | Before FDA Query | After Resolution |
|---|---|---|
| CPP Definition | Partial | Fully defined with supporting risk assessments |
| PAT Utilization | Not included | Inline monitoring for blend uniformity |
| Residual Solvent Specs | Generic ICH levels | Product-specific justifications added |
These changes showed improved process control and a deeper understanding of the manufacturing process.
Writing Clear and Structured FDA Responses
All responses followed a three-part format: a summary answer, detailed explanation, and supporting data. Tables, charts, and NDA cross-references made the responses easier for reviewers to read and understand.
This writing style helped the FDA quickly assess each response, reducing the chance of follow-up questions or rejections.
Get expert medicinal chemistry support for your startup: Outsourced Medicinal Chemistry for Startups
Using Real-World Evidence (RWE)
Where internal data was limited, real-world evidence (RWE) from scientific literature was used. This included published impurity data and manufacturing information for similar drug products.
All references came from peer-reviewed journals or trusted regulatory sources. This added weight to the sponsor’s justifications and showed alignment with current standards.
Communicating Proactively with the FDA
To avoid misunderstandings, the sponsor requested a teleconference with the FDA to walk through key responses. This early conversation helped clarify assumptions and reduced the risk of further queries.
Proactive communication built trust with the review team and demonstrated the sponsor’s commitment to transparency.
Meeting Timelines Without Extensions
All responses to FDA queries were submitted within 30 calendar days, with no request for extra time. A modular strategy was used so that urgent issues could be submitted first.
Advanced planning ensured that all documents were in the correct format and ready for electronic submission (eCTD). Despite tight deadlines, the sponsor met all quality standards and regulatory expectations.
Accelerate your drug discovery timelines with our CRO services: Learn about CRO Chemistry Timelines for Drug Discovery
Outcome: NDA Approved Without Delays
The FDA accepted all responses, and no further CMC issues were noted in the final review. The NDA was approved within the original timeline, and no Complete Response Letter was issued.
This case confirms that a clear strategy, early planning, and expert regulatory knowledge are essential for resolving FDA CMC queries efficiently and successfully.
Key Takeaways from Resolving FDA CMC Queries
- Simulating FDA reviews early can help avoid major setbacks.
- Scientific data bridging can fill in gaps and avoid new studies.
- Clear, structured communication improves decision-making speed.
- Cross-functional teamwork ensures consistent and timely responses.
- Experienced experts are vital for solving technical challenges effectively.
Looking for a partner in Canada to handle your CMC challenges? Explore our Drug Discovery CRO Services in Canada
📌 Frequently Asked Questions (FAQs)
FDA CMC queries refer to questions from the Food and Drug Administration related to the Chemistry, Manufacturing, and Controls section of a New Drug Application (NDA). These questions are aimed at verifying product quality, consistency, and regulatory compliance before approval.
Generally, the FDA allows up to 30 calendar days to respond to CMC queries. In certain situations, companies may request an extension, but timely and accurate responses are essential to avoid potential delays in the approval process.
Analytical method validation is a critical part of the FDA’s review, especially for impurity control and product stability. The FDA expects clear evidence that the method is reliable, accurate, and suitable for its intended purpose.
Cross-functional teams bring together expertise from different departments such as regulatory, quality, manufacturing, and analytical development. This collaboration helps ensure that responses are accurate, consistent, and submitted on time.
Real-world evidence can be very useful when internal data is limited. It provides context by showing how similar drugs or processes perform in real settings, strengthening the sponsor’s justification and regulatory case.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas