Article Summary
- Outsourced Chemistry in Drug Discovery plays a critical execution role between target validation and IND-enabling studies
- Strategic outsourcing accelerates hit-to-lead, lead optimization, and candidate selection
- External chemistry partners de-risk timelines through parallel synthesis, scalable routes, and early developability insight
- IND success depends on chemistry decisions made far earlier than many teams realize
- The right outsourcing model balances scientific continuity, IP protection, and regulatory readiness
Introduction: Why Outsourced Chemistry Determines IND Readiness Earlier Than You Think
Outsourced Chemistry in Drug Discovery has become a defining factor in how efficiently drug ideas move from early concepts to IND-enabling studies. As pipelines grow more complex and timelines shrink, chemistry execution often becomes the deciding factor between progress and failure. Chemistry is no longer just a support function; it directly shapes outcomes.
While biological targets often receive the most attention, it is chemistry execution that determines whether a promising concept can become a viable clinical candidate. Poor synthetic routes, unstable compounds, or unscalable processes can stop a program, even when biology looks strong. Early chemistry discipline helps avoid these common pitfalls.
Today, most discovery teams cannot manage the full chemistry workload internally. Instead, outsourced chemistry acts as an extension of internal scientific leadership, influencing molecular design, scalability, manufacturability, and regulatory confidence from an early stage. These partners provide both execution power and strategic insight.
This article focuses on how Outsourced Chemistry in Drug Discovery supports the journey from idea generation to IND-enabling studies. Rather than offering generic outsourcing definitions, it explains where, when, and why chemistry outsourcing directly affects success.
Explore specialized support for your drug discovery milestones: Drug Discovery CRO Chemistry Services
How Outsourced Chemistry Fits Into the Idea-to-IND Continuum
Outsourced Chemistry in Drug Discovery supports the entire early pipeline by turning conceptual molecules into IND-ready assets through structured and continuous execution. Each discovery phase places different demands on chemistry, and outsourcing helps meet those needs without slowing progress. The objective is continuity, not disconnected handoffs.
From the first medicinal chemistry hypothesis to reproducible, GMP-aligned routes, outsourced chemistry influences every downstream milestone. Early decisions affect toxicology readiness, formulation options, and manufacturing feasibility. Strong chemistry foundations reduce late-stage surprises.
External chemistry teams often bring experience across multiple development phases. This allows them to anticipate how early design choices may impact later IND requirements. Such long-term perspective is difficult to maintain when chemistry is treated as a series of isolated tasks.
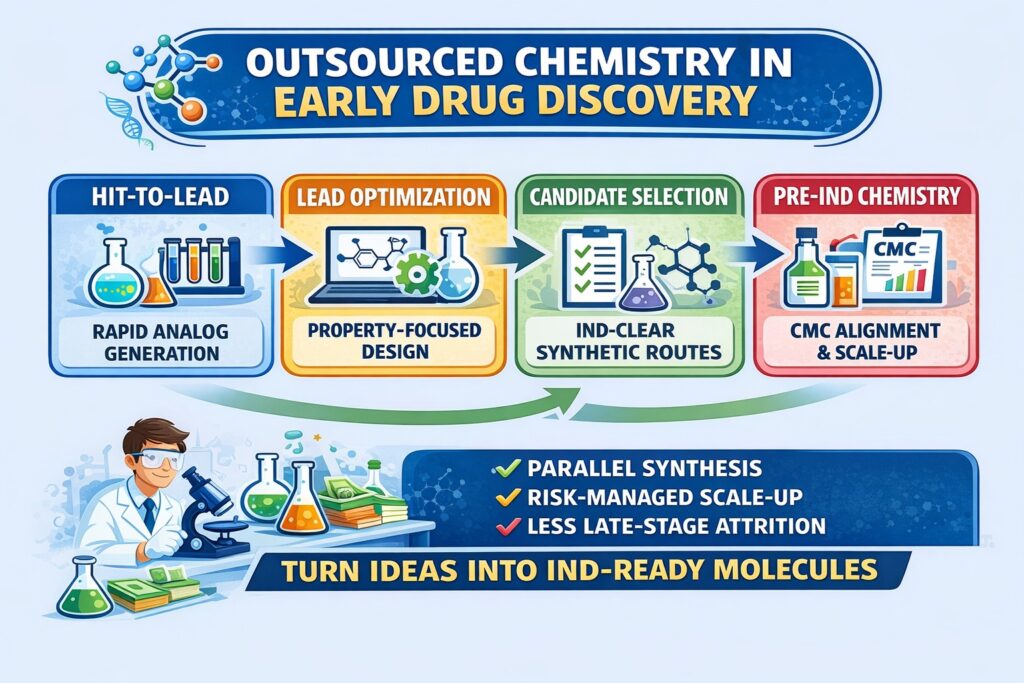
Key Early-Stage Phases Where Outsourced Chemistry Is Mission-Critical
| Drug Discovery Phase | Role of Outsourced Chemistry | Impact on IND Readiness |
|---|---|---|
| Hit-to-Lead | Rapid analog generation, SAR exploration | Establishes chemical tractability |
| Lead Optimization | Property tuning, metabolic stability | Reduces late-stage attrition |
| Candidate Selection | Route robustness, impurity profiling | Enables confident nomination |
| Pre-IND Chemistry | Scale-up & CMC alignment | Prevents IND delays |
Outsourcing today is no longer transactional. When partners are engaged early and consistently, Outsourced Chemistry in Drug Discovery becomes a strategic driver of program momentum and risk reduction.
Discover how a virtual team can extend your internal capabilities: Virtual Chemistry Department CRO
Hit-to-Lead: Where Outsourced Chemistry Accelerates Decision-Making
Outsourced Chemistry in Drug Discovery significantly accelerates hit-to-lead timelines by enabling parallel synthesis and faster SAR cycles. Speed at this stage is critical, as early programs often compete for internal funding and strategic focus. Outsourcing allows teams to test more ideas in less time.
This phase demands fast output without sacrificing data quality. External chemistry teams can handle large synthesis volumes while maintaining strong analytical standards. This ensures decisions are based on reliable and reproducible data rather than assumptions.
Outsourced partners also help teams avoid early commitment to chemically weak scaffolds. By quickly generating alternatives, they preserve flexibility and reduce the risk of dead-end chemistry paths. This adaptability is essential when biology is still evolving.
What Outsourced Chemistry Enables at Hit-to-Lead
- Rapid creation of focused analog libraries
- Parallel testing of multiple synthetic routes
- Early identification of instability or reactivity risks
- Faster go/no-go decisions using solid chemical data
Experienced partners do more than make compounds. They help identify chemotypes that are more likely to survive lead optimization and IND scrutiny, saving valuable time and resources.
Accelerate your hit-to-lead phase with expert synthesis: Medicinal Chemistry Services for Drug Discovery
Lead Optimization: Designing With IND in Mind, Not Just Potency
Outsourced Chemistry in Drug Discovery delivers maximum value during lead optimization, where chemistry decisions directly influence IND success. At this stage, programs move from exploration to refinement, and each change has long-term consequences.
Potency alone is not enough to advance a candidate. Solubility, stability, metabolic behavior, and synthetic scalability are equally important. Outsourced chemistry teams help balance these factors while keeping programs moving forward. Their experience supports informed trade-offs.
This is also the phase where regulatory expectations quietly enter the picture. Chemistry partners familiar with IND standards can guide molecule design choices that align with future CMC needs, even before formal IND work begins.
Outsourced Chemistry Contributions During Lead Optimization
- Property-focused design (clearance, lipophilicity, permeability)
- Scaffold refinement to reduce toxicology risk
- Early impurity and degradation pathway analysis
- Simplified routes that support future scale-up
By designing with IND expectations in mind, outsourced chemistry partners reduce the need for late-stage redesigns and help maintain development timelines.
Refine your lead candidates with advanced metabolite insights: Metabolite Synthesis Services for Drug Discovery
Candidate Selection: Chemistry as a Gatekeeper to IND Success
Outsourced Chemistry in Drug Discovery plays a decisive role during candidate selection, determining whether a molecule can realistically progress into IND-enabling studies. At this point, chemistry must support confident, data-driven decisions.
The focus shifts from “can we make it” to “can we make it reliably, safely, and at scale.” Outsourced chemistry teams provide objective evaluations based on real-world experience rather than theoretical assumptions.
Many programs fail at this stage due to overlooked chemistry risks. Early identification of these issues prevents expensive delays and loss of credibility later.
Chemistry Criteria That Influence Candidate Nomination
- Reproducible and robust synthetic routes
- Availability and cost of raw materials
- Feasibility of impurity control strategies
- Salt selection and solid-state behavior
Strong outsourced chemistry support helps teams avoid choosing biologically exciting but chemically impractical candidates, one of the most common causes of late-stage failure.
Transition from discovery to development with reliable synthesis: Custom Synthesis for Drug Discovery
IND-Enabling Studies Start Earlier Than the IND
Outsourced Chemistry in Drug Discovery effectively begins IND-enabling work long before the formal IND stage. Many challenges encountered during IND preparation are rooted in early discovery chemistry decisions. Chemistry connects all development phases.
Choices made during discovery directly impact toxicology studies, stability programs, and manufacturing campaigns. Weak routes or unstable compounds increase regulatory risk and complicate execution. Early chemistry rigor prevents these issues.
Outsourced teams help embed IND awareness into discovery workflows without slowing innovation. This balance supports both speed and long-term quality.
Early Chemistry Decisions That Affect IND-Enabling Studies
- Selection of scalable and reliable synthetic routes
- Control strategies for known and potential impurities
- Preliminary stability and stress testing
- Compatibility with toxicology formulations
Early outsourcing ensures IND-enabling studies confirm progress rather than correct past mistakes, saving time, cost, and effort.
Ensure your analytical methods meet rigorous industry standards: Custom Analytical Method Development for Drug Discovery
Why Experience in Outsourced Chemistry Matters More Than Capacity
Outsourced Chemistry in Drug Discovery is only as effective as the expertise behind it. Simply increasing capacity does not solve complex chemistry challenges. Experience, judgment, and foresight matter far more than volume.
Poor execution may deliver compounds quickly but fail under IND scrutiny. Experienced teams anticipate regulatory and manufacturing expectations early, reducing downstream risk.
For small and mid-sized biotech companies, outsourcing provides access to senior-level chemistry insight without the burden of building large internal teams. This flexibility is a major advantage.
Characteristics of High-Value Chemistry Partners
- Strong integration of medicinal and process chemistry
- Clear understanding of pre-IND regulatory expectations
- Transparent documentation and data integrity
- Willingness to challenge assumptions constructively
True expertise is shown by preventing problems before they occur, not reacting after timelines slip.
Tailored chemistry expertise for emerging biotech and startups: Outsourced Medicinal Chemistry for Startups
Managing Risk, IP, and Knowledge Continuity Through Outsourcing
Outsourced Chemistry in Drug Discovery must be structured carefully to protect intellectual property and maintain scientific continuity. Without proper governance, outsourcing can introduce fragmentation instead of efficiency.
Clear roles, consistent communication, and shared standards are essential. When managed well, outsourcing reduces risk rather than increasing it.
Continuity becomes especially important as programs move between discovery and development stages. Long-term chemistry partners help preserve critical knowledge.
Best Practices for Risk-Managed Chemistry Outsourcing
- Clearly defined data ownership and IP boundaries
- Centralized documentation for compounds and routes
- Regular communication between internal and external teams
- Structured and transparent decision-making processes
When executed correctly, outsourced chemistry becomes a seamless extension of internal discovery efforts.
How Outsourced Chemistry Aligns With AI-Driven Drug Discovery Models
Outsourced Chemistry in Drug Discovery plays a key role in AI-driven discovery by converting digital predictions into physical molecules. AI can suggest designs, but chemistry proves whether they work in reality.
Computational insights only create value when compounds can be synthesized, tested, and optimized efficiently. Outsourced chemistry provides this execution layer, keeping feedback loops fast and actionable.
Partners experienced in AI-guided workflows help bridge the gap between in silico design and IND-ready molecules, strengthening both chemistry and AI outcomes.
Maintain momentum with predictable and efficient project timelines: CRO Chemistry Services Timelines for Drug Discovery
Conclusion: Outsourced Chemistry as a Strategic Lever From Idea to IND
Outsourced Chemistry in Drug Discovery is not a late-stage convenience. It is a strategic lever that determines whether ideas progress smoothly to IND-enabling studies or fail early. Its influence begins at hit-to-lead and continues through candidate selection.
Programs that succeed typically integrate chemistry thinking early and maintain it consistently across phases. Outsourcing done right supports speed, quality, and regulatory confidence.
Organizations that treat outsourced chemistry as a true scientific partnership consistently achieve faster timelines, lower risk, and stronger IND packages. This approach turns chemistry execution into a competitive advantage.
Contact ResolveMass Today – Contact Us
Frequently Asked Questions (FAQs)
Outsourced chemistry should ideally start at the hit-to-lead stage. Early involvement helps teams quickly test ideas, confirm synthetic feasibility, and avoid weak chemical paths. This early support reduces rework later and creates a stronger foundation for IND-enabling studies.
Yes, outsourced chemistry has a direct impact on IND success. Many IND delays are caused by early issues with synthesis routes, impurities, or compound stability. Addressing these topics early through experienced partners improves regulatory confidence.
IND-relevant outsourced chemistry focuses on scalable routes, impurity control, and reproducible data. It also includes proper documentation that supports regulatory review. These elements ensure smoother progress into IND-enabling studies.
Outsourcing chemistry is not inherently risky when managed correctly. Clear contracts, defined ownership, and strong data controls protect IP. In many cases, structured partnerships reduce risk compared to informal internal workflows.
Outsourced chemistry speeds development by enabling parallel synthesis and faster SAR cycles. It also identifies chemical risks early, preventing delays during late-stage development. This results in more predictable and efficient timelines.
Regulators closely review impurity profiles, compound stability, and route reproducibility. They also expect clear control strategies and consistent documentation. Early chemistry planning helps meet these expectations with confidence.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196


