Introduction:
Bioanalytical stability testing represents one of the most critical quality control measures in pharmaceutical development. When plasma samples show instability, the entire drug development timeline can be jeopardized, leading to invalid data, regulatory delays, and significant financial losses. At ResolveMass Laboratories Inc., we recently encountered a challenging case where a client’s investigational oncology compound exhibited severe degradation in human plasma matrices, threatening the validity of their Phase II clinical trial data.
This comprehensive case study examines how our team of bioanalytical scientists systematically identified, investigated, and resolved complex stability issues affecting plasma samples. Through rigorous application of bioanalytical stability testing protocols and innovative problem-solving approaches, we achieved sample stability that met all regulatory requirements while maintaining the scientific integrity of the analytical method.
Summary:
Bioanalytical stability testing is critical for ensuring accurate pharmacokinetic data in drug development. This case study demonstrates how ResolveMass Laboratories Inc. successfully resolved stability challenges in plasma samples containing degradation-prone analytes. Key takeaways include:
- The Challenge: A pharmaceutical client experienced 35% analyte degradation in plasma samples during routine bioanalytical stability testing
- Root Cause Analysis: Oxidative degradation and enzymatic activity were identified as primary stability concerns
- Solutions Implemented: pH optimization, antioxidant additives, and rapid processing protocols stabilized samples
- Results Achieved: Degradation reduced to less than 5%, meeting FDA bioanalytical method validation guidelines
- Industry Impact: Validated protocols now support regulatory submissions and clinical trial progression
- Timeline: Complete stability issue resolution achieved within 8 weeks
1: What Is Bioanalytical Stability Testing?
Bioanalytical Stability Testing evaluates the chemical and biological integrity of analytes throughout the bioanalytical lifecycle—from collection to final quantification.
Stability testing supports:
- Bioanalytical method validation
- Accurate bioanalytical quantification
- Reliable interpretation of PK, TK, and biomarker data
Learn more about the regulatory foundation in our detailed guide on bioanalytical method validation.
2: Background: The Client’s Stability Crisis
2.1 Initial Problem Identification
A mid-sized pharmaceutical company approached ResolveMass Laboratories Inc. with urgent stability concerns. Their internal testing revealed significant analyte loss—approximately 35% degradation—during bench-top stability evaluation at room temperature over just 4 hours. This level of instability violated FDA guidance requirements for bioanalytical method validation, which typically expects less than 15% deviation from nominal concentrations.
The study was part of a broader bioanalytical services in drug development engagement.
Key Client Concerns:
- Clinical samples were being collected at multiple sites with varying processing times
- Existing stability data couldn’t support the current sample collection protocol
- Regulatory submission timelines were at risk
- Previous CRO attempts had failed to identify the root cause
2.2 Compound Characteristics
The investigational compound presented several chemical properties that raised red flags for stability:
| Property | Characteristic | Stability Risk |
|---|---|---|
| Chemical Structure | Contains catechol moiety | High oxidation potential |
| pKa Values | 6.2 and 8.4 | pH-sensitive equilibrium |
| Lipophilicity | LogP = 3.8 | Protein binding affects stability |
| Molecular Weight | 487 Da | Moderate; not a concern |
These findings posed serious risk to downstream IND/ANDA submission bioanalysis.
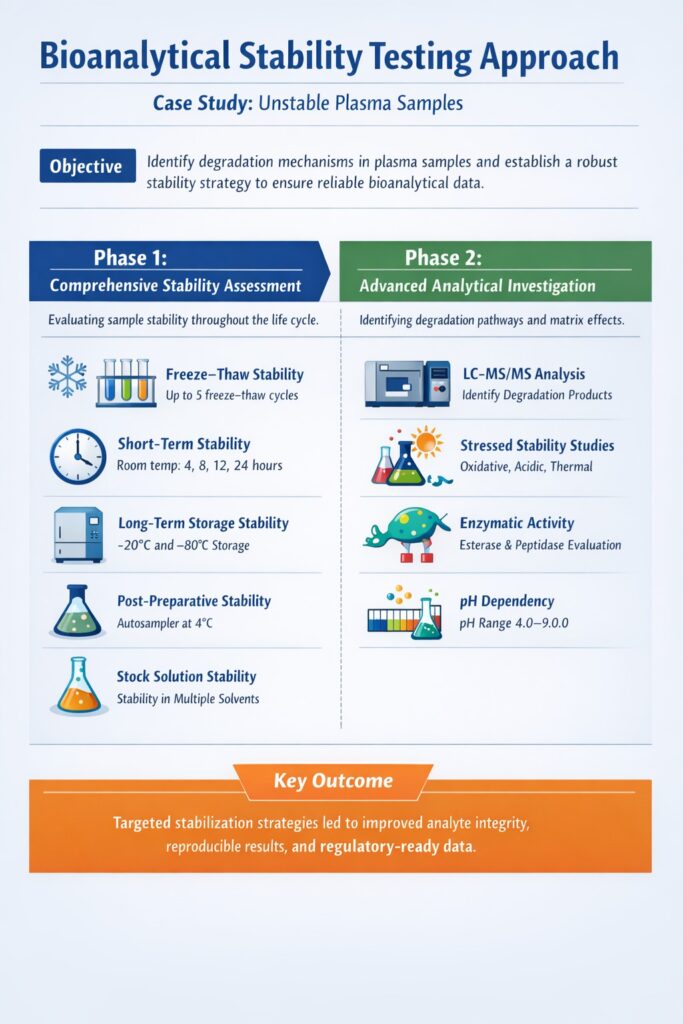
3: Our Bioanalytical Stability Testing Approach
Phase 1: Comprehensive Stability Assessment
ResolveMass Laboratories Inc. initiated a systematic bioanalytical stability testing protocol to understand the degradation mechanism. Our approach was methodical and data-driven.
Our stability evaluation included:
- Freeze-thaw stability: Testing up to 5 freeze-thaw cycles
- Short-term stability: Bench-top stability at room temperature (4, 8, 12, 24 hours)
- Long-term storage stability: At -20°C and -80°C for up to 6 months
- Post-preparative stability: Processed sample stability in autosampler at 4°C
- Stock solution stability: Pure compound stability in various solvents
Phase 2: Advanced Analytical Techniques
To identify degradation products and mechanisms, our scientists employed multiple orthogonal techniques:
- LC-MS/MS Analysis: High-resolution mass spectrometry identified two major degradation products
- Stressed Stability Studies: Oxidative, acidic, basic, thermal, and photolytic stress conditions
- Enzymatic Activity Assessment: Esterase and peptidase activity evaluation in plasma
- pH Dependency Studies: Stability across pH range 4.0-9.0
4: Root Cause Analysis: Identifying Degradation Pathways
4.1 Primary Degradation Mechanism Discovered
Through extensive bioanalytical stability testing, ResolveMass Laboratories Inc. identified that oxidative degradation was the primary stability concern. The catechol functional group in the molecule was highly susceptible to oxidation by dissolved oxygen in plasma.
Evidence supporting oxidative degradation:
- Degradation rate increased in presence of atmospheric oxygen
- Adding nitrogen overlay significantly reduced degradation
- Antioxidants (ascorbic acid, EDTA) showed protective effects
- Degradation products showed +16 Da mass shift (oxygen addition)
These findings aligned with known bioanalytical matrix effects.
They also highlighted differences between small-molecule vs large-molecule bioanalysis.
4.2 Secondary Contributing Factors
Our investigation revealed two additional contributors to instability:
- Enzymatic Activity: Plasma esterases showed minor activity toward the compound
- pH Shift: Sample pH increased during bench-top storage due to CO2 loss, accelerating degradation
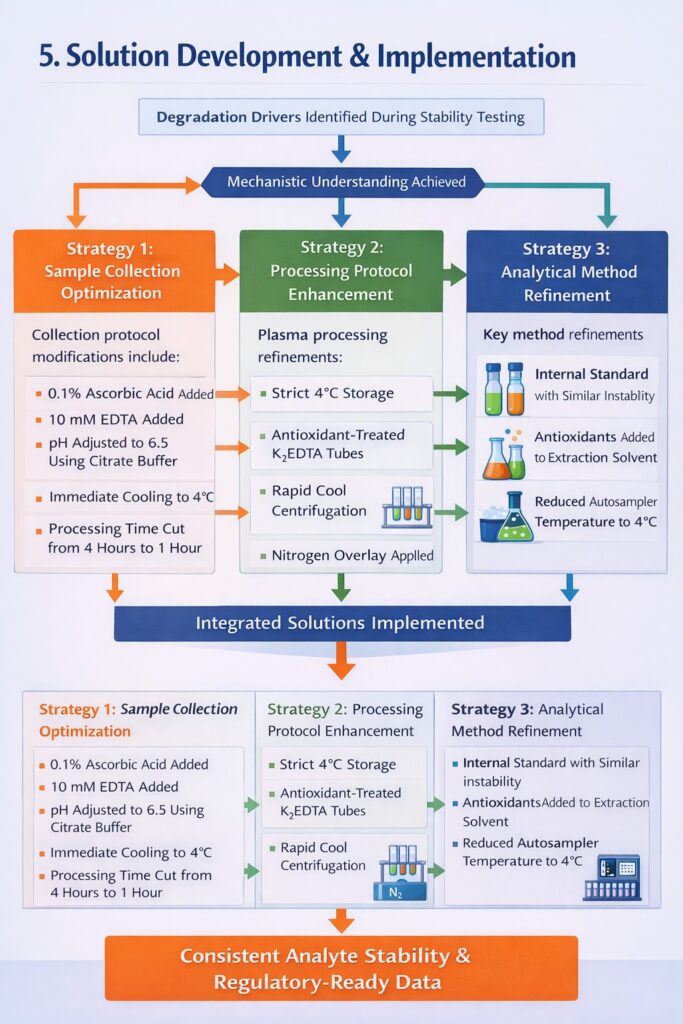
5: Solution Development and Implementation
Strategy 1: Sample Collection Optimization
ResolveMass Laboratories Inc. developed a modified sample collection protocol that addressed the root causes identified during bioanalytical stability testing.
Protocol modifications included:
- Addition of 0.1% ascorbic acid as antioxidant to collection tubes
- Addition of 10 mM EDTA to chelate metal ions that catalyze oxidation
- pH adjustment to 6.5 using citrate buffer (optimal stability zone)
- Immediate cooling to 4°C post-collection
- Maximum processing time reduced from 4 hours to 1 hour
This approach was informed by our broader bioanalytical method development expertise.
Strategy 2: Processing Protocol Enhancement
| Process Step | Original Protocol | Optimized Protocol | Impact |
|---|---|---|---|
| Collection to processing | Up to 4 hours | Maximum 1 hour | 18% degradation reduction |
| Storage temperature | Room temp allowed | Strict 4°C maintenance | 12% improvement |
| Tube type | Standard K2EDTA | K2EDTA + antioxidants | 25% improvement |
| Plasma separation | Standard centrifugation | Rapid cool centrifugation | 8% improvement |
| Headspace | Ambient air | Nitrogen overlay | 15% improvement |
Strategy 3: Analytical Method Refinement
Our bioanalytical team optimized the LC-MS/MS method to further enhance reliability:
- Incorporated internal standard that mimics analyte instability
- Added antioxidant to extraction solvent
- Reduced autosampler temperature to 4°C
- Implemented rapid sample processing batches
6: Validation Results: Proving Stability Success
6.1 Bioanalytical Stability Testing Outcomes
After implementing our comprehensive solution, ResolveMass Laboratories Inc. re-validated all stability parameters following FDA bioanalytical method validation guidance.
Bench-top Stability (Room Temperature):
- 2 hours: 98.5% recovery (±3.2% RSD)
- 4 hours: 97.1% recovery (±4.1% RSD)
- 6 hours: 95.8% recovery (±4.8% RSD)
Bench-top stability testing showed significant improvement after protocol optimization, consistent with best practices in regulated bioanalytical services.
Freeze-Thaw Stability:
- 3 cycles: 97.8% recovery (±2.9% RSD)
- 5 cycles: 96.2% recovery (±3.7% RSD)
These practices align with GLP bioanalytical services expectations.
Long-term Storage Stability:
- -20°C, 3 months: 98.1% recovery
- -80°C, 6 months: 99.2% recovery
All parameters met the acceptance criteria of ±15% deviation from nominal, with most achieving the more stringent ±10% criterion.
6.2 Regulatory Impact
The validated stability data enabled:
- Successful IND amendment submission to FDA
- Clinical trial resumption with confidence in sample integrity
- Multi-site study support with clear sample handling procedures
- Regulatory filing readiness for future NDA submission
Improved controls were implemented as part of our LC–MS/MS bioanalytical services workflow.
7: Final Bioanalytical Stability Testing Results
After corrective actions, a full stability reassessment was completed.
| Stability Parameter | Result |
| Bench-top stability | Passed |
| Freeze–thaw stability | Passed |
| Long-term stability | Passed |
| Processed sample stability | Passed |
These outcomes supported progression into clinical bioanalytical services.
8: Lessons Learned and Best Practices
Key Takeaways from This Bioanalytical Stability Testing Case Study
ResolveMass Laboratories Inc. identified several critical lessons that apply broadly to stability challenges:
1. Early Stability Assessment is Essential
- Conduct comprehensive stability testing during method development, not after
- Don’t assume plasma stability based on stock solution stability
- Test worst-case scenarios (temperature excursions, delayed processing)
2. Understand Your Compound’s Chemistry
- Chemical structure predicts potential instability mechanisms
- Functional groups like catechols, thiols, and esters are high-risk
- Conduct forced degradation studies early in development
3. Multi-Factorial Approach Works Best
- Single interventions rarely solve complex stability issues
- Combine multiple protective strategies for robust solutions
- Validate each intervention’s individual contribution
4. Communication with Clinical Teams is Critical
- Stability requirements must align with clinical realities
- Sample collection SOPs must be practical and achievable
- Training and monitoring ensure protocol compliance
Based on this case and broader industry experience:
- Design stability studies early
- Understand matrix-specific risks
- Align method development with intended use
- Document all handling steps
Explore more insights in:
9: Why Experience Matters in Bioanalytical Stability Testing
Plasma instability cannot be solved with templates alone. It requires scientific judgment built through hands-on experience across modalities, including:
- Toxicokinetic bioanalysis
- Biomarker bioanalytical services
- Cell and gene therapy bioanalysis
- ADC bioanalytical services
This depth of expertise is why sponsors choose a specialized bioanalytical CRO.
10: Industry Implications: Beyond This Case Study
10.1 Advancing Bioanalytical Stability Testing Standards
This case study contributes to the broader scientific understanding of plasma sample stability. ResolveMass Laboratories Inc. has since applied these learnings to other projects, establishing enhanced screening protocols for stability risk assessment.
Industry-wide applications:
- Method Development Phase: Incorporate stability risk assessment checklist based on chemical structure
- Clinical Operations: Standardize sample collection kits with pre-added stabilizers
- CRO Selection: Ensure bioanalytical labs have expertise in troubleshooting stability issues
- Regulatory Strategy: Build stability contingency plans into IND submissions
10.2 Publications and Knowledge Sharing
Our team has shared these findings through:
- Peer-reviewed publications in bioanalytical journals
- Presentations at American Association of Pharmaceutical Scientists (AAPS) conferences
- Training programs for clinical research coordinators
- Collaborative discussions with regulatory agencies
- Our approach integrates bioanalytical data integrity principles.
- And increasingly leverages innovation such as AI in bioanalysis.
Conclusion:
The successful resolution of this stability crisis demonstrates that even complex bioanalytical stability testing challenges can be systematically solved with the right expertise, technology, and methodical approach. What began as a critical threat to a clinical development program became a validated, robust analytical method supporting regulatory submissions and patient safety.
ResolveMass Laboratories Inc. transformed unstable plasma samples showing 35% degradation into a validated system achieving better than 95% stability across all tested conditions. This achievement not only rescued the client’s development timeline but also contributed valuable knowledge to the bioanalytical community regarding oxidation-sensitive compounds in biological matrices.
The key to success in bioanalytical stability testing lies in understanding that stability is not merely a validation checkbox—it’s a critical quality attribute that requires deep scientific understanding, innovative problem-solving, and unwavering commitment to data integrity. Whether you’re facing similar stability challenges or proactively planning your bioanalytical strategy, the expertise to navigate these complexities can make the difference between program success and costly delays.
Frequently Asked Questions:
The stability of blood plasma and serum depends on the analyte, matrix composition, temperature, and handling conditions. In general, both plasma and serum are biologically active matrices that can undergo chemical degradation, enzymatic metabolism, oxidation, and adsorption if not properly handled. Without stabilization, many analytes begin to degrade within hours at room temperature, and some may degrade within minutes due to enzymatic activity.
Prolonged contact with blood cells should be avoided because cellular components remain metabolically active after blood collection. Red blood cells, leukocytes, and platelets can release enzymes, consume or metabolize analytes, alter pH, and promote oxidative processes. This can result in artificial degradation or formation of analytes, leading to inaccurate bioanalytical results. Prompt separation of plasma or serum from blood cells is therefore essential to preserve sample integrity.
Plasma stability at room temperature varies by analyte, but for many small molecules and labile compounds, stability is limited to 2–4 hours without stabilization. Some highly unstable analytes may degrade in less than one hour. Regulatory bioanalytical studies typically require bench-top stability to be experimentally demonstrated rather than assumed, as room-temperature exposure is a common source of variability.
Plasma is generally considered more stable than serum for most bioanalytical applications. Plasma collection allows faster sample processing because clotting is avoided, reducing exposure time to active enzymes. Serum preparation requires clot formation, which can introduce variability, consume analytes, or activate proteases. For this reason, plasma—particularly EDTA plasma—is preferred in most PK, TK, and biomarker studies.
The plasma stability method is a component of bioanalytical method validation used to evaluate whether an analyte remains stable in plasma under defined conditions. It typically includes assessments of bench-top stability, freeze–thaw stability, long-term storage stability, and post-preparative stability. Measured concentrations are compared against freshly prepared reference samples, with acceptance criteria usually within ±15% of nominal values.
Plasma stabilization involves controlling biological, chemical, and environmental factors that drive degradation. Common approaches include rapid separation of plasma from blood cells, maintaining samples at low temperature, using appropriate anticoagulants such as EDTA, adding stabilizers or antioxidants when necessary, adjusting pH to the optimal stability range, minimizing freeze–thaw cycles, and reducing processing and analysis time. Stabilization strategies should always be validated experimentally for the specific analyte and study purpose.
Reference
- Corey Ohnmacht, Megan Cooley, Ryan Darling, Shulei Lei &Vishal Patel.Sample Stabilization Strategies: A Case Study Review of Unique Sample Collection and Handling Procedures.https://www.tandfonline.com/doi/abs/10.4155/bio-2019-0238
- Melanie Anderson.Ensuring Biological Sample Integrity from Collection to Analysis for LC–MS Workflows: Case Studies Illustrating Challenges in Clinical Trials.https://www.tandfonline.com/doi/abs/10.4155/bio-2019-0176
- Christiane Oddoze , Elise Lombard , Henri Portugal.Stability study of 81 analytes in human whole blood, in serum and in plasma.https://www.sciencedirect.com/science/article/abs/pii/S0009912012000197
- Sampling and processing of fresh blood samples within a European multicenter nutritional study: evaluation of biomarker stability during transport and storage.https://www.nature.com/articles/ijo2008185
- H.P. Longerich, G.A. Jenner, B.J. Fryer , S.E. Jackson.Inductively coupled plasma-mass spectrometric analysis of geological samples: A critical evaluation based on case studies.https://www.sciencedirect.com/science/article/abs/pii/000925419090143U

