🔍 Summary of Key Insights
- Outsourced medicinal chemistry enabled faster hit-to-lead and lead optimization.
- Integrated partner collaboration helped reduce cycle times by 40%.
- Compound libraries were expanded 3x through external synthesis capabilities.
- Structure–activity relationship (SAR) insights accelerated decision-making.
- Outsourcing minimized internal resource strain, allowing core team focus.
- The project delivered a viable preclinical candidate 6 months ahead of projection.
- Cost optimization through scalable FTE models.
- Deep alignment between client discovery scientists and external chemists proved critical.
Introduction: The Value of Outsourced Medicinal Chemistry Case Study
How can pharmaceutical companies speed up lead optimization without compromising data integrity or intellectual property?
This Outsourced Medicinal Chemistry Case Study explores how a stalled discovery program evolved into a preclinical success through smart outsourcing.
By expanding their capabilities through a trusted external partner, the client gained operational speed and flexibility without losing scientific depth.
The example proves that outsourcing can be a strategic growth lever rather than just a temporary fix.
Explore our core solutions: Drug Discovery Chemistry Services
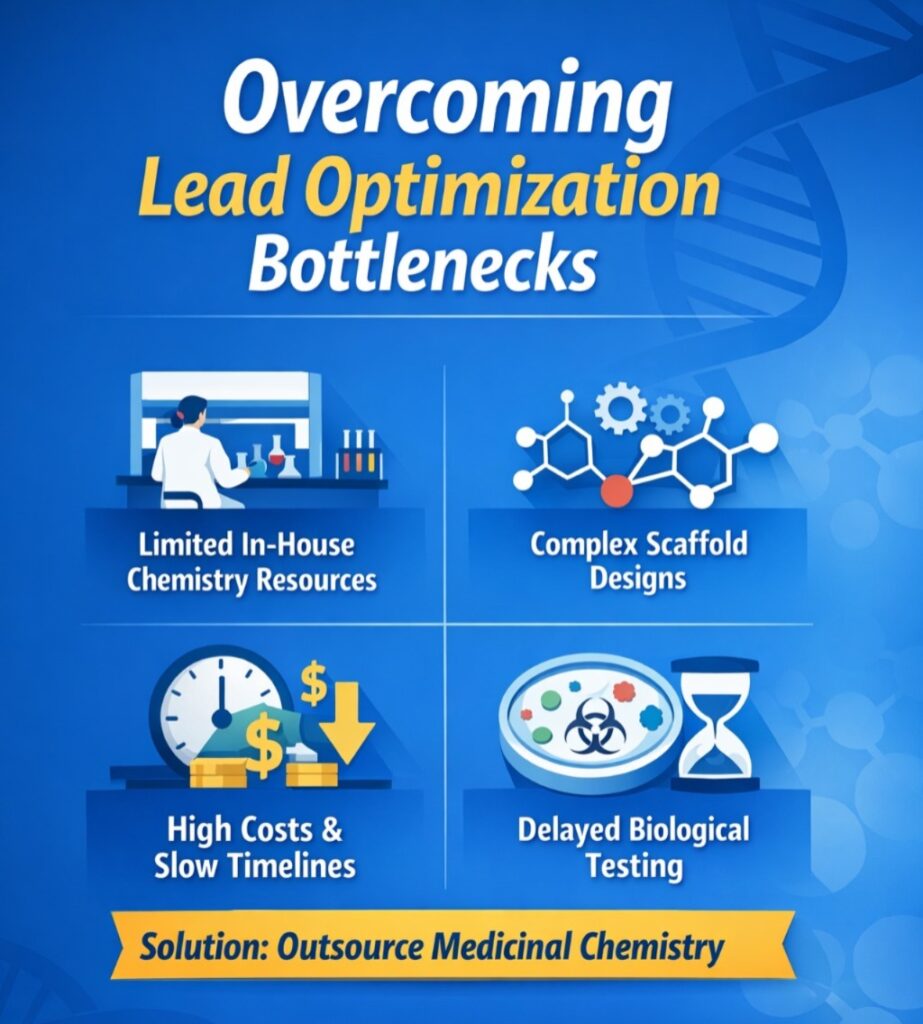
The Challenge: Addressing Lead Optimization Bottlenecks
The client—a mid-sized biopharma company—faced repeated delays during hit-to-lead progression. The root cause? A highly complex chemical scaffold and limited in-house chemistry bandwidth.
Despite promising hits against a novel kinase target, the team couldn’t generate analogs fast enough to keep up with biological testing. The complexity of multi-step synthesis slowed SAR cycles and delayed decisions, increasing the overall project risk.
❌ Key Obstacles Faced:
- Limited internal synthetic chemistry resources preventing parallel analog development
- Complex scaffold structures requiring specialized synthesis expertise
- High internal costs due to iterative testing cycles
- Delays in biological feedback due to slow compound delivery
Overcome synthesis bottlenecks: Custom Synthesis for Drug Discovery
Strategic Decision: Choosing to Outsource Medicinal Chemistry
Why was outsourcing the ideal solution? The company needed to scale its chemistry efforts fast—without expanding internal headcount.
Partnering with an external medicinal chemistry provider brought in specialized chemists, infrastructure, and the ability to conduct synthesis in parallel.
The focus was on seamless integration into ongoing workflows, all while protecting sensitive IP and ensuring transparency.
✅ What the Client Looked for in a Partner:
- Proven experience with kinase inhibitors and complex scaffolds
- Transparent communication and collaborative project management
- Strong IP protection protocols
- Flexible FTE-based engagement model
- Advanced purification, analysis, and characterization tools
Scale your team efficiently: Outsourced Medicinal Chemistry for Startups
Execution Phase: Speeding Up Through Strategic Outsourcing
Within just four weeks, the outsourced chemistry team synthesized more than 60 analogs—triple what the internal team achieved in three months.
Using a fully integrated FTE model, the external team aligned with the client’s SAR strategy daily.
Design, synthesis, and feedback occurred in parallel, enabling fast responses to biological data and course corrections as needed.
🔬 Workflow Breakdown Table:
| Phase | Duration | Outcome |
|---|---|---|
| Onboarding & Handoff | Week 1 | Rapid technology transfer and scaffold briefing |
| Analog Design & Prioritization | Weeks 2–3 | Parallel design sessions using AI tools |
| Synthesis & Characterization | Weeks 3–6 | 60+ compounds delivered at >85% purity |
| SAR Review & Feedback Loop | Ongoing | Weekly SAR reviews with real-time reprioritization |
Accelerate your project timelines: CRO Chemistry Services Timelines for Drug Discovery
AI-Driven Compound Prioritization for Medicinal Chemistry
To streamline decision-making, the outsourced team utilized AI-based retrosynthesis planning. These tools helped rank analogs based on their likelihood of biological activity, ease of synthesis, and novelty.
AI tools also minimized duplication and ensured that IP risks were flagged early.
Key Benefits of AI Integration:
- Fewer unnecessary synthesis cycles
- Higher density of SAR data in shorter time
- Increased novelty, reducing IP overlap risks
Results: Clear Gains from Outsourced Medicinal Chemistry
This Outsourced Medicinal Chemistry Case Study showed tangible performance improvements. Speed, cost, and output all improved significantly under the outsourced model.
Internal teams could concentrate on data interpretation and translational strategy, while synthesis was handled externally.
📈 Impact Summary Table:
| Metric | Internal Team | Outsourced Team | Gain |
|---|---|---|---|
| Analog Synthesis (first 6 weeks) | 18 compounds | 64 compounds | +255% |
| Cycle Time per Analog | 10–14 days | 3–5 days | ~60% faster |
| Time to Viable Lead | 9 months | 3.5 months | 61% faster |
| Cost per Compound | $6,500 avg. | $3,200 avg. | 50% savings |
Optimize your R&D budget: Cost-Effective Drug Discovery Chemistry
How the Collaboration Enabled Real-Time Agility
Success was not driven by technical ability alone—it was the collaboration model that made the difference. Both internal and outsourced teams worked within a shared communication system, giving everyone real-time access to data and progress updates.
Collaboration Best Practices:
- Daily Slack updates for compound status
- Shared cloud storage for all data
- Joint SAR meetings every five working days
- Complete retrosynthesis documentation for traceability
Seamlessly integrate external expertise: Virtual Chemistry Department CRO
IP Protection and Regulatory Readiness
IP security and compliance were embedded into the process from the beginning. The outsourced partner maintained strict data protocols and lab controls. Each compound batch had full traceability, ensuring downstream regulatory and CMC readiness.
Security and Compliance Measures:
- Encrypted data transmission pipelines
- Controlled-access lab environments for IP-sensitive work
- Full traceability of all synthesized compounds
- OECD GLP-compliant documentation
Ensure data integrity and precision: Custom Analytical Method Development for Drug Discovery
Lessons from This Outsourced Medicinal Chemistry Case Study
This case offered several key takeaways for successfully outsourcing medicinal chemistry. Cost-saving alone wasn’t enough—strategic alignment and communication mattered even more.
Key Lessons:
- Integrate the teams — external chemists should function as part of the internal group
- Define early goals — clarify SAR objectives and hit criteria from the start
- Use AI wisely — for compound design and synthesis planning
- Set communication rhythms — regular updates prevent delays
- Focus on expertise — especially for complex synthetic challenges
- Plan ahead — for documentation and regulatory needs
Conclusion: Strategic Wins from Outsourced Medicinal Chemistry
This Outsourced Medicinal Chemistry Case Study proves that outsourcing can be a strategic growth tool, not just a cost-saving move.
By partnering with an experienced external provider and integrating workflows, the client achieved faster optimization cycles and smarter decisions.
ResolveMass Laboratories helps clients unlock similar value through customized outsourcing solutions.
When your internal resources are stretched or timelines are tight, outsourcing may be the boost your pipeline needs.
Partner with a leading Canadian CRO: Drug Discovery CRO in Canada
Let’s explore how ResolveMass Laboratories can support your drug discovery goals:
FAQs on Outsourced Medicinal Chemistry Case Studies
Outsourcing significantly speeds up lead optimization by allowing more compounds to be synthesized and tested in parallel. With scalable external resources, timelines can be shortened by up to 2–3 times compared to internal efforts alone.
Yes, reliable outsourcing partners implement strong data protection practices. These include non-disclosure agreements, encrypted systems, and restricted access to sensitive project information to safeguard intellectual property.
Intellectual property is secured through clear legal agreements and strict lab protocols. Ownership clauses are included in contracts, and all compound data is stored securely with full documentation to ensure client rights are protected.
Complex small molecules such as kinase inhibitors, heterocycles, and compounds requiring multi-step synthesis are well-suited for outsourcing. These projects benefit from the specialized skills and infrastructure available through external partners.
Yes, outsourced teams can work closely with internal biology teams using shared communication platforms and synchronized review cycles. This ensures smooth coordination and real-time integration of chemistry and biology data.
An FTE (Full-Time Equivalent) model means dedicated chemists work exclusively on your project. This approach offers continuity, faster progress, and clear accountability compared to ad hoc outsourcing methods.
Reference
- Shareef, U., Altaf, A., Ahmed, M., Akhtar, N., Almuhayawi, M. S., Al Jaouni, S. K., Selim, S., Abdelgawad, M. A., & Nagshabandi, M. K. (2023). A comprehensive review of discovery and development of drugs discovered from 2020–2022. Saudi Pharmaceutical Journal, 32(1), 101913. https://doi.org/10.1016/j.jsps.2023.101913
- U.S. Food and Drug Administration. (n.d.). Drug development and review definitions. U.S. Department of Health and Human Services. Retrieved February 6, 2026, from https://www.fda.gov/drugs/investigational-new-drug-ind-application/drug-development-and-review-definitions
- Patel, P., Sharma, V., & Meshram, D. B. (2024). A review on drug design and development. International Journal of Pharmacognosy and Pharmaceutical Research, 6(2), 08–20. https://doi.org/10.33545/26647168.2024.v6.i2a.72
- Hughes, J. P., Rees, S., Kalindjian, S. B., & Philpott, K. L. (2011). Principles of early drug discovery. British Journal of Pharmacology, 162(6), 1239–1249. https://doi.org/10.1111/j.1476-5381.2010.01127.x