
Introduction
High sensitivity bioanalysis has become the cornerstone of modern pharmaceutical development, particularly when evaluating low-dose drug candidates that require detection at sub-nanogram concentrations. At ResolveMass Laboratories Inc., we understand that achieving sub-nanogram sensitivity isn’t merely about having sophisticated instrumentation—it’s about implementing a comprehensive analytical strategy that combines cutting-edge technology, expert method development, and rigorous quality control across the entire drug development lifecycle. Our approach is grounded in proven bioanalytical services for drug development and supported by a full spectrum of bioanalytical laboratory services.
Summary
Key Takeaways:
- Sub-nanogram sensitivity in high sensitivity bioanalysis enables detection of drug concentrations below 1 ng/mL, critical for low-dose therapeutic development and clinical bioanalytical service
- Advanced LC-MS/MS techniques combined with optimized sample preparation enable accurate bioanalytical quantification
- Proper bioanalytical method validation ensures regulatory-ready PK and TK data
- Strategic instrumentation selection supports both small molecule vs large molecule bioanalysis
- ResolveMass Laboratories Inc. provides cost-effective, scalable solutions including affordable bioanalytical services for biotech startups
1: Understanding Sub-Nanogram Sensitivity Requirements
Sub-nanogram sensitivity refers to the ability to accurately quantify analytes below 1 ng/mL in biological matrices using validated high sensitivity bioanalysis workflows. This capability is critical for modern therapeutics such as antibody-drug conjugates, oligonucleotides, and cell & gene therapies, which often require specialized platforms like LC-MS bioanalysis for oligonucleotides and cell and gene therapy bioanalysis.
High sensitivity bioanalysis ensures accurate PK/PD bioanalysis and supports early discovery through IND and NDA submission stages.
Why Sub-Nanogram Detection Matters:
- Enables evaluation of highly potent compounds with low therapeutic doses
- Supports extended pharmacokinetic profiling with longer sampling timepoints
- Facilitates assessment of metabolites and degradation products at trace levels
- Meets regulatory requirements for comprehensive safety and efficacy evaluation
2: Advanced Instrumentation for High Sensitivity Bioanalysis
Achieving sub-nanogram sensitivity requires state-of-the-art mass spectrometry platforms optimized for trace-level quantification. The choice of instrumentation directly impacts the lower limit of quantification (LLOQ) and overall method performance.
2.1 Triple Quadrupole Mass Spectrometry
Triple quadrupole (QqQ) LC-MS/MS systems remain the gold standard for high sensitivity bioanalysis. These instruments achieve exceptional sensitivity through highly selective multiple reaction monitoring (MRM), which isolates precursor ions in the first quadrupole, fragments them in the collision cell, and selects specific product ions in the third quadrupole. This triple-stage filtration dramatically reduces chemical background noise.
Triple quadrupole LC-MS/MS systems remain the gold standard for regulated LC-MS/MS bioanalytical services and LC-MS/MS bioanalysis of xenobiotics.
These platforms are central to regulated GLP bioanalytical services and high-throughput bioanalysis.
Key Performance Features:
| Instrument Parameter | Impact on Sensitivity |
|---|---|
| Ion source design | Determines ionization efficiency and spray stability |
| Quadrupole mass resolution | Controls selectivity and noise reduction |
| Collision cell technology | Affects fragmentation efficiency and sensitivity |
| Detector sensitivity | Sets ultimate detection limits |
2.2 High-Resolution Mass Spectrometry (HRMS)
While traditional QqQ systems excel at targeted quantification, high-resolution accurate mass (HRAM) instruments like Orbitrap and Q-TOF systems offer complementary advantages. HRMS platforms provide mass accuracy typically below 5 ppm, enabling highly specific detection that can differentiate target analytes from isobaric interferences.
HRMS is increasingly used for complex modalities such as biosimilar bioanalysis and antibody-drug conjugate bioanalytical services.
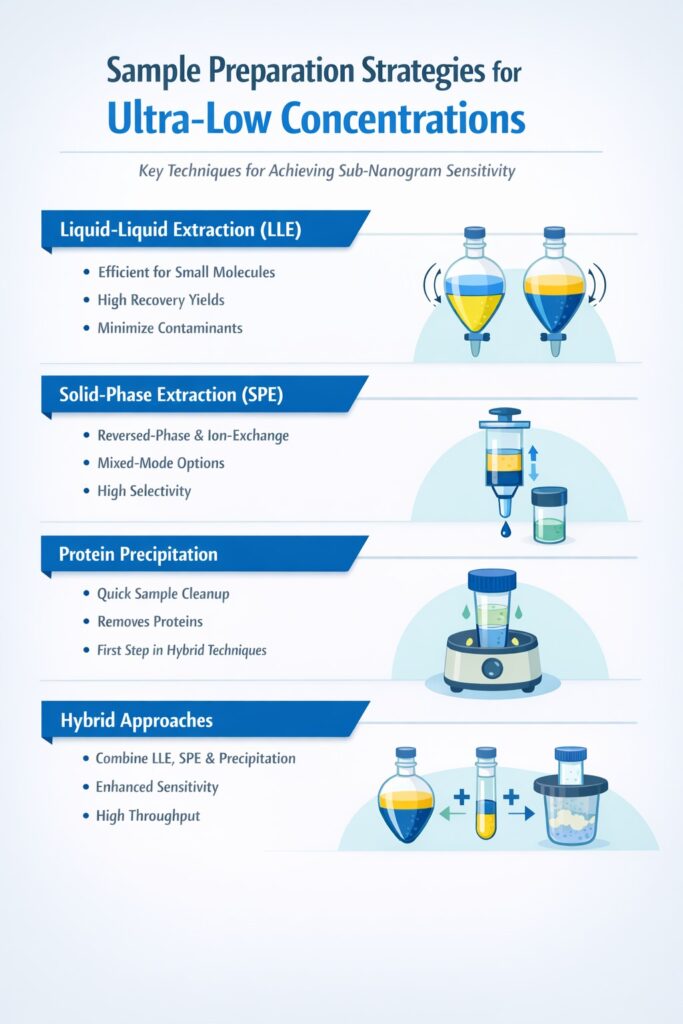
3: Sample Preparation Strategies for Ultra-Low Concentrations
Sample preparation represents the critical first step in high sensitivity bioanalysis. Even the most advanced mass spectrometer cannot compensate for poor sample extraction or excessive matrix effects.
Effective sample preparation is foundational to managing bioanalytical matrix effects and overcoming challenges commonly encountered during bioanalytical method development.
Hybrid workflows improve sensitivity while supporting scalable bioanalytical outsourcing and outsourced bioanalysis for drug development.
3.1 Liquid-Liquid Extraction (LLE)
Liquid-liquid extraction provides excellent cleanup for many small molecule applications. By selecting appropriate organic solvents, analysts can achieve high recovery while removing interfering matrix components. For sub-nanogram work, special attention must be paid to background contamination from solvents, glassware, and plasticware.
3.2 Solid-Phase Extraction (SPE)
Solid-phase extraction offers superior selectivity through various retention mechanisms:
- Reversed-phase SPE for hydrophobic analytes
- Ion-exchange SPE for charged compounds
- Mixed-mode SPE combining multiple interactions
- Immunoaffinity SPE for ultimate selectivity
SPE Optimization Checklist:
- Evaluate multiple sorbent chemistries during method development
- Optimize washing steps to remove matrix interferences without analyte loss
- Use LC-MS grade solvents to minimize background contamination
- Implement positive controls to monitor recovery throughout sample sets
3.3 Protein Precipitation
While protein precipitation alone rarely achieves the cleanup necessary for sub-nanogram quantification, it serves as an effective first step in hybrid approaches. Combining protein precipitation with subsequent SPE or LLE can provide both high throughput and excellent sensitivity.
4: Chromatographic Considerations in High Sensitivity Bioanalysis
Sharp, well-resolved chromatographic peaks are essential for achieving optimal sensitivity. Peak dispersion dilutes analyte concentration, directly reducing signal intensity and degrading detection limits.
Optimized chromatography is essential for minimizing noise and improving sensitivity in both regulated bioanalytical services and discovery-stage workflows such as discovery vs regulated bioanalysis.
Critical Chromatographic Parameters:
- Column selection: Smaller particle sizes (sub-2 μm) and shorter columns (50 mm) reduce band broadening
- Mobile phase optimization: High organic content and appropriate pH enhance ionization efficiency
- Flow rate: Balance between resolution and sensitivity, typically 0.3-0.5 mL/min for sensitivity-critical applications
- Injection volume: Maximum possible without compromising peak shape, often 10-50 μL for bioanalytical applications
5: Mass Spectrometer Optimization for Maximum Sensitivity
Achieving sub-nanogram sensitivity through high sensitivity bioanalysis requires meticulous optimization of every mass spectrometer parameter.
Instrument tuning directly impacts the success of rapid bioanalytical method development and long-term method robustness across clinical programs.
5.1 Ionization Source Optimization
Electrospray ionization (ESI) efficiency depends on numerous factors:
- Source temperature balances desolvation efficiency with thermal degradation
- Spray voltage affects droplet formation and ion generation
- Nebulizing gas pressure influences spray stability
- Probe positioning impacts ion transmission into the mass analyzer
5.2 Compound-Specific Tuning
Each analyte requires individual optimization through direct infusion experiments:
- Precursor ion selection: Identify the most abundant ionized form ([M+H]+, [M+Na]+, etc.)
- Collision energy optimization: Maximize product ion formation for selected transitions
- Transition selection: Choose the most sensitive and specific MRM transitions
- Dwell time allocation: Distribute acquisition time to maximize signal-to-noise ratios
6: Method Validation for Sub-Nanogram Quantification
Regulatory agencies require comprehensive validation of bioanalytical methods before accepting study data. For high sensitivity bioanalysis at sub-nanogram levels, validation presents unique challenges.
Sub-nanogram assays require rigorous bioanalytical method development and validation with documented bioanalytical data integrity.
Validation supports toxicokinetic bioanalysis and IND/NDA-ready submissions.
6.1 Lower Limit of Quantification (LLOQ)
The LLOQ must demonstrate:
- Signal-to-noise ratio ≥5 (preferably ≥10)
- Accuracy within ±20% of nominal concentration
- Precision ≤20% CV
- Reproducibility across multiple batches and days
6.2 Calibration Curve Performance
Sub-nanogram methods typically employ:
- Concentration ranges spanning 3-4 orders of magnitude
- Weighted linear regression (1/x² or 1/x) to account for heteroscedasticity
- Minimum 6-8 calibration standards plus blank samples
- Internal standard correction for ion suppression/enhancement
Validation Parameters Specific to High Sensitivity Bioanalysis:
| Parameter | Acceptance Criteria | Special Considerations |
|---|---|---|
| Accuracy | ±15% (±20% at LLOQ) | Verify across multiple operators |
| Precision | ≤15% CV (≤20% at LLOQ) | Include long-term reproducibility assessment |
| Selectivity | No interference >20% of LLOQ | Test multiple blank matrices |
| Matrix effects | Ion suppression/enhancement <25% | Evaluate post-column infusion |
| Recovery | Consistent across concentration range | Not required to be 100% |
| Stability | Covers anticipated study conditions | Include autosampler, freeze-thaw, long-term |
7: Overcoming Common Challenges in Sub-Nanogram Detection
Challenges such as contamination and instability are addressed through bioanalytical stability testing and advanced digital solutions including AI in bioanalysis.
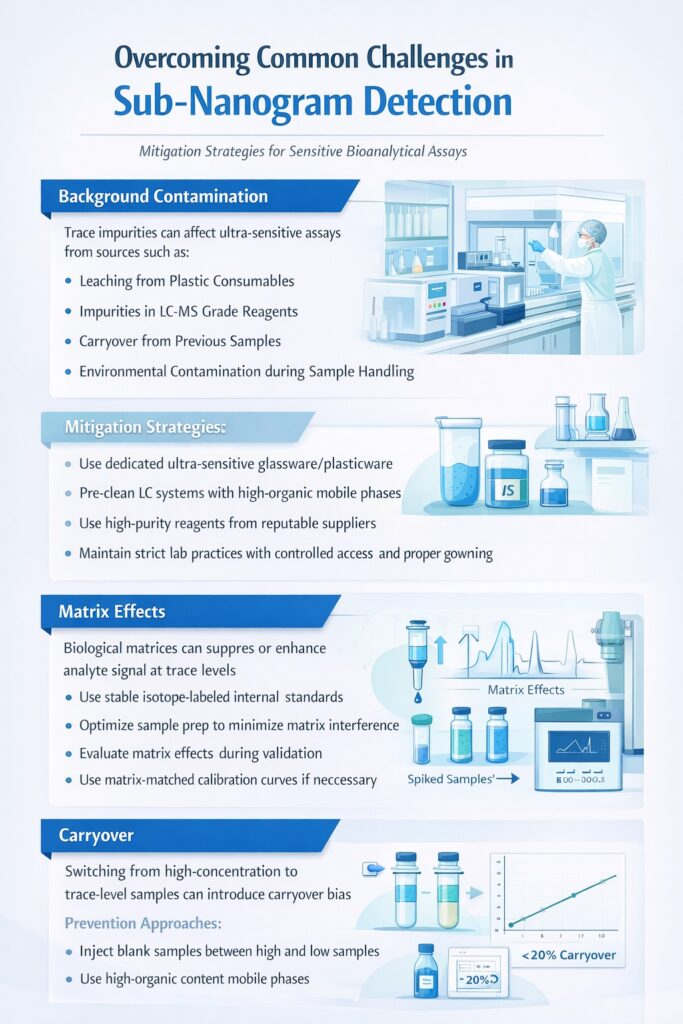
7.1 Background Contamination
Trace contamination represents the primary obstacle in ultra-sensitive high sensitivity bioanalysis. Sources include:
- Leaching from plastic consumables
- Impurities in LC-MS grade reagents
- Carryover from previous samples
- Environmental contamination during sample handling
Mitigation Strategies:
- Implement dedicated glassware and plasticware for ultra-sensitive methods
- Pre-clean LC systems with high-organic mobile phases
- Use high-purity reagents from reputable suppliers
- Establish strict laboratory practices including controlled access and proper gowning
7.2 Matrix Effects
Biological matrices contain thousands of endogenous compounds that can suppress or enhance analyte ionization. At sub-nanogram concentrations, even minor ion suppression significantly impacts quantification accuracy.
Solutions:
- Employ stable isotope-labeled internal standards that co-elute with analytes
- Optimize sample preparation to maximize matrix removal
- Evaluate matrix effects during method validation using post-extraction addition experiments
- Consider matrix-matched calibration curves when necessary
7.3 Carryover
When analyzing samples at sub-nanogram levels immediately after high-concentration samples, carryover can cause significant bias.
Prevention Approaches:
- Inject blank samples between high-concentration standards and unknowns
- Implement thorough needle wash protocols with strong and weak wash solutions
- Use high-organic content mobile phases to flush the LC system
- Establish maximum acceptable carryover limits during validation (typically <20% of LLOQ)
8: ResolveMass Laboratories Inc.: Your Partner in Ultra-Sensitive Bioanalysis
At ResolveMass Laboratories Inc., we’ve built our reputation on delivering reliable high sensitivity bioanalysis for the most challenging pharmaceutical applications. Our expert team combines decades of mass spectrometry experience with state-of-the-art instrumentation to achieve picogram-level quantification when your program demands it.
ResolveMass operates as a specialized bioanalytical CRO supporting virtual biotech models and guiding sponsors through bioanalytical CRO vs in-house decisions.
Our Comprehensive Capabilities Include:
- Method development and validation following FDA, EMA, and ICH guidelines
- Quantification of small molecules, peptides, proteins, and biomarkers
- Support for preclinical through Phase III clinical studies
- Rapid turnaround times without compromising data quality
- Regulatory-ready documentation and audit support
Our services include:
- Bioanalytical CRO services for PK and TK
- Managing bioanalytical CRO projects
- Cost-effective bioanalytical services
Full service overview:
https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
https://resolvemass.ca/bioanalytical-services/
We understand that achieving sub-nanogram sensitivity requires more than equipment—it demands scientific expertise, attention to detail, and unwavering commitment to quality. Our laboratory infrastructure includes dedicated ultra-sensitive suites with environmental controls, comprehensive quality systems, and continuous method performance monitoring.
9: Regulatory Considerations for Ultra-Sensitive Methods
When developing high sensitivity bioanalysis methods for regulatory submission, adherence to current guidelines is paramount. The FDA’s Bioanalytical Method Validation Guidance (2018) and EMA’s Guideline on Bioanalytical Method Validation provide frameworks, but ultra-sensitive methods often require additional considerations.
High sensitivity bioanalysis underpins why bioanalysis is important in regulatory science and supports advanced modalities such as proteomics bioanalytical services.
Key Regulatory Expectations:
- Demonstrate that the LLOQ is appropriate for the intended purpose
- Provide scientific justification for all method parameters
- Include incurred sample reanalysis (ISR) to verify method reliability
- Document any method deviations or modifications with appropriate justification
- Maintain comprehensive records supporting method development decisions
10: Future Trends in High Sensitivity Bioanalysis
The field of ultra-sensitive bioanalysis continues to evolve rapidly. Emerging technologies promise even lower detection limits:
- Microflow and nanoflow LC-MS: Reduced flow rates concentrate analytes for enhanced sensitivity
- Acoustic ejection mass spectrometry (AEMS): Label-free detection with minimal sample consumption
- Ion mobility spectrometry (IMS): Additional separation dimension reduces chemical noise
- Automated sample preparation: Robotics improve reproducibility while reducing contamination risk
- Digital microfluidics: Miniaturized sample handling for ultra-low volume applications
Conclusion
Achieving sub-nanogram sensitivity for low-dose drug candidates through high sensitivity bioanalysis represents a significant analytical challenge that requires expertise, advanced technology, and meticulous attention to detail. From instrument selection and optimization to sample preparation and method validation, every step must be carefully executed to ensure reliable quantification at these ultra-low concentration levels. As pharmaceutical development increasingly focuses on highly potent therapeutics, the demand for sub-nanogram bioanalytical capabilities will only intensify. ResolveMass Laboratories Inc. stands ready to support your most demanding bioanalytical needs with proven expertise in high sensitivity bioanalysis, delivering the accurate, reproducible data essential for successful drug development programs.
Frequently Asked Questions :
High Sensitivity Bioanalysis refers to analytical techniques capable of detecting and quantifying drugs and metabolites at sub-nanogram or even picogram concentrations in complex biological matrices.
Sub-nanogram sensitivity is essential because low-dose drugs produce extremely low plasma or tissue concentrations, especially during early clinical trials and microdosing studies.
The most common challenges include background contamination, matrix effects, carryover, analyte instability, and poor extraction recovery at ultra-low concentrations.
Sample preparation directly influences sensitivity by controlling matrix interference and analyte loss. Poor preparation can negate the benefits of advanced LC–MS/MS instrumentation.
Solid-phase extraction (SPE) is most commonly preferred due to its high selectivity, though hybrid workflows combining protein precipitation with SPE or LLE often deliver optimal results.
Matrix effects are caused by co-eluting endogenous compounds that suppress or enhance ionization during LC–MS analysis, significantly affecting accuracy at low concentrations.
Reference
- Jolitta S. J. Britto, Xinwei Guan, Thi Kim Anh Tran, Zhihao Lei, Rohan Bahadur, Vaishwik Patel, Xiangwei Zhang, Sharon L. Wong, Ajayan Vinu. Emerging Multifunctional Carbon-Nanomaterial-Based Biosensors for Cancer Diagnosis.https://onlinelibrary.wiley.com/doi/full/10.1002/smsc.202300221
- The Stage-specific Testicular Germ Cell Apoptotic Response to Low-dose X-irradiation and 2,5-hexanedione Combined Exposure. I: Validation of the Laser Capture Microdissection Method for qRT-PCR Array Application.https://journals.sagepub.com/doi/full/10.1177/0192623314526319
- A High-Volume, High-Throughput LC–MS Therapeutic Drug Monitoring System.https://www.chromatographyonline.com/view/high-volume-high-throughput-lc-ms-therapeutic-drug-monitoring-system-0
- P Teale, J Scarth &S Hudson.Impact of The Emergence of Designer Drugs Upon Sports Doping Testing.https://www.tandfonline.com/doi/abs/10.4155/bio.11.291

