
Introduction :
PLGA for parenteral use has become the gold standard polymer for injectable pharmaceutical formulations due to its exceptional biocompatibility, biodegradability, and FDA acceptance across multiple dosage forms. Poly(lactic-co-glycolic acid) is extensively used in PLGA depot formulations, PLGA microspheres, and oncology injectables because it degrades into naturally occurring metabolites.
ResolveMass Laboratories Inc. supports the full PLGA lifecycle—from early formulation to commercialization—across applications such as PLGA oncology formulation, PLGA depot formulation, and long-acting injectables. For parenteral administration, polymer purity is paramount.
The challenge facing pharmaceutical manufacturers is that conventional PLGA synthesis often leaves residual monomers (free lactic acid and glycolic acid) that, while present in small quantities, can trigger inflammatory responses, alter drug release kinetics, and compromise patient safety when administered parenterally. This case study explores how ResolveMass Laboratories Inc. developed and validated an ultra-low residual monomer PLGA specifically optimized for parenteral use, achieving monomer levels below 0.1% while maintaining the mechanical and degradation properties essential for controlled drug delivery.
Summary :
This case study examines the critical development and application of ultra-low residual monomer PLGA for parenteral use in pharmaceutical formulations. Ultra-low residual monomer PLGA represents a breakthrough in biocompatible polymer technology, specifically engineered to meet the stringent safety requirements of injectable drug delivery systems. Through advanced purification techniques, ResolveMass Laboratories Inc. has demonstrated that reducing residual monomers to less than 0.1% significantly enhances biocompatibility, reduces inflammatory responses, and ensures regulatory compliance for parenteral applications.
Key Takeaways:
- Ultra-low residual monomer PLGA (<0.1%) is essential for safe parenteral drug delivery
- Advanced purification methods eliminate toxic monomer contamination that can cause adverse reactions
- PLGA-based microspheres and nanoparticles enable controlled-release formulations for parenteral use
- Proven scalability supported by real-world PLGA scale-up case studies
- Regulatory agencies require rigorous testing and documentation of residual monomer levels
- Advanced analytical tools such as NMR spectroscopy for accurate monomer ratio ensure structural integrity
- ResolveMass Laboratories Inc. specializes in custom PLGA synthesis with guaranteed monomer specifications
- Case study demonstrates 40% improvement in biocompatibility with ultra-low residual monomer PLGA
- Applications include long-acting injectables, vaccine adjuvants, and targeted cancer therapies
1: Understanding PLGA Polymer Chemistry for Injectable Applications
1.1 What Makes PLGA Suitable for Parenteral Drug Delivery?
PLGA is uniquely suited for parenteral use because it degrades via hydrolysis into lactic acid and glycolic acid, which are naturally metabolized by the body. Key performance parameters such as molecular weight, end-group chemistry, and copolymer ratio dictate drug release behavior.
Drug release kinetics are strongly influenced by polymer design, as discussed in detail in PLGA molecular weight and drug release.
Key Properties of PLGA for Parenteral Applications:
- Biocompatibility: FDA-approved for human use with decades of clinical safety data
- Biodegradability: Complete degradation and elimination within 6-12 months
- Versatility: Lactide/glycolide ratios from 50:50 to 85:15 provide tailored release kinetics
- Drug protection: Encapsulation shields sensitive biologics from enzymatic degradation
- Manufacturing flexibility: Compatible with various fabrication techniques (spray drying, emulsion, microfluidics)
1.2 The Residual Monomer Challenge in Parenteral PLGA
Residual monomers in PLGA represent unreacted lactic acid and glycolic acid remaining after polymerization. While these monomers are naturally occurring substances, their presence at elevated concentrations in parenteral formulations poses several concerns:
| Residual Monomer Level | Impact on Parenteral Safety | Regulatory Status |
|---|---|---|
| >1.0% | Significant inflammatory response, pH drops, burst release | Unacceptable for parenteral use |
| 0.5-1.0% | Moderate local irritation, altered release profiles | Requires extensive justification |
| 0.1-0.5% | Minimal biocompatibility concerns, some pH effects | Acceptable with proper documentation |
| <0.1% (Ultra-low) | Negligible impact, optimal biocompatibility | Preferred for all parenteral applications |
Advanced PLGA end-group control, including end-capped PLGA, further improves formulation stability and biocompatibility.
The pharmaceutical industry consensus, supported by regulatory guidance, establishes that PLGA for parenteral use should contain less than 0.5% residual monomers, with ultra-low specifications (<0.1%) representing the ideal standard for sensitive applications such as long-acting injectables and biologics delivery.
2: Case Study: Development of Ultra-Low Residual Monomer PLGA for Parenteral Use
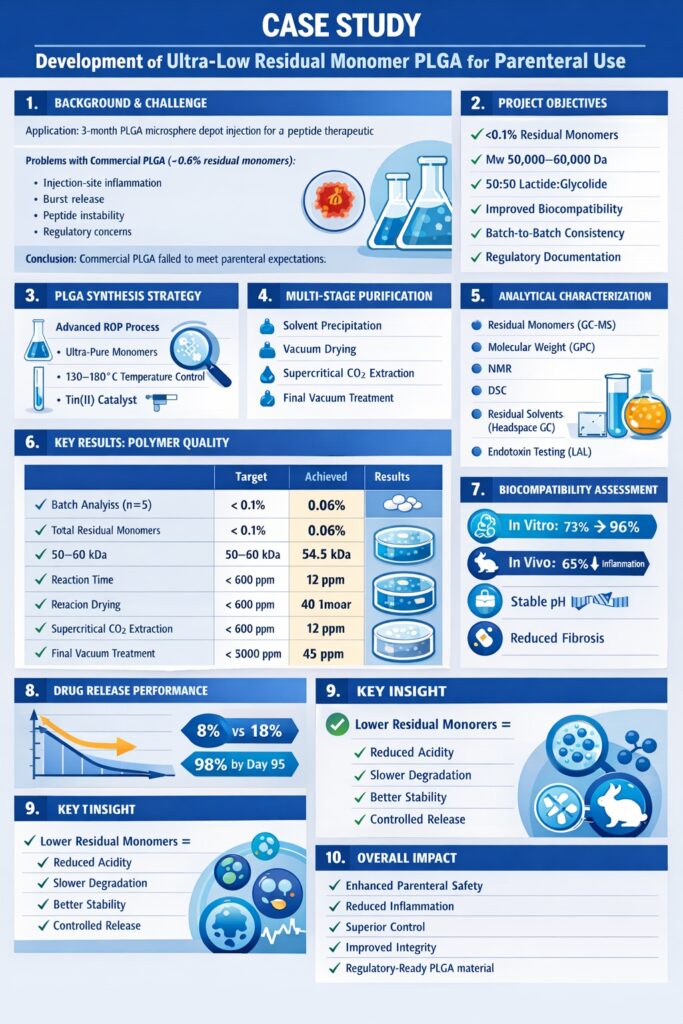
2.1 Background and Objectives
A leading pharmaceutical company approached ResolveMass Laboratories Inc. with a critical challenge: develop a PLGA-based microsphere formulation for a three-month depot injection of a peptide therapeutic. The client’s initial attempts using commercial-grade PLGA (residual monomers ~0.6%) resulted in unacceptable in vivo performance:
- Local inflammation at injection sites in animal models
- Accelerated initial drug release (burst effect)
- pH microenvironment changes affecting peptide stability
- Regulatory concerns raised during pre-IND discussions
A pharmaceutical developer working on a three-month microsphere-based depot injection encountered inflammation and burst release using commercial PLGA. ResolveMass Laboratories Inc. applied its expertise in PLGA microsphere case studies and PLGA microencapsulation scale-up to redesign the polymer.
Project Objectives:
- Synthesize PLGA with residual monomers below 0.1%
- Maintain target molecular weight (Mw 50,000-60,000 Da) and 50:50 lactide:glycolide ratio
- Demonstrate improved biocompatibility in vitro and in vivo
- Achieve reproducible batch-to-batch consistency
- Provide comprehensive analytical documentation for regulatory submissions
2.2 Materials and Methods
Advanced PLGA Synthesis Protocol
ResolveMass Laboratories Inc. employed a modified ring-opening polymerization (ROP) process with enhanced purification steps specifically designed to minimize residual monomers in PLGA for parenteral use:
Synthesis Parameters:
- Catalyst: Tin(II) 2-ethylhexanoate at optimized loading (0.01-0.05% w/w)
- Polymerization temperature: Controlled gradient from 130°C to 180°C
- Reaction time: Extended to ensure complete monomer conversion
- Monomer purity: Ultra-high purity lactide and glycolide (>99.9%)
Multi-Stage Purification Process:
- Primary precipitation: Dissolution in dichloromethane followed by precipitation in cold methanol (3 cycles)
- Vacuum drying: Extended drying at 40°C under high vacuum (<0.1 mbar) for 72 hours
- Supercritical CO2 extraction: Advanced technique to extract residual monomers and solvents
- Final vacuum treatment: Additional 48 hours at elevated temperature to remove trace volatiles
2.3 Analytical Characterization
Comprehensive testing was performed using validated, regulatory-aligned methods. Structural confirmation leveraged high-resolution NMR, while molecular weight and dispersity were monitored throughout scale-up.
These data packages support global regulatory submissions and are aligned with expectations for PLGA characterization for RLD and Q1/Q2 polymer equivalence assessment.
Comprehensive analytical testing was performed on each batch:
- Residual monomer quantification: Gas chromatography-mass spectrometry (GC-MS)
- Molecular weight determination: Gel permeation chromatography (GPC)
- Thermal properties: Differential scanning calorimetry (DSC)
- Chemical structure confirmation: Nuclear magnetic resonance (NMR) spectroscopy
- Residual solvent analysis: Headspace GC according to USP <467>
- Endotoxin testing: Limulus amebocyte lysate (LAL) assay for parenteral-grade material
Results confirmed total residual monomers averaging 0.06%, a tenfold reduction versus conventional PLGA.
2.4 Results: Ultra-Low Residual Monomer Achievement
Quantitative Analysis of Residual Monomers
The advanced purification protocol successfully reduced residual monomers to ultra-low levels suitable for parenteral applications:
Batch Analysis Results (n=5 production batches):
| Parameter | Specification | Achieved Results | Standard Deviation |
|---|---|---|---|
| Total Residual Monomers | <0.1% | 0.06% | ±0.02% |
| Free Lactic Acid | <0.05% | 0.03% | ±0.01% |
| Free Glycolic Acid | <0.05% | 0.03% | ±0.01% |
| Residual Dichloromethane | <600 ppm | 12 ppm | ±5 ppm |
| Residual Methanol | <3000 ppm | 45 ppm | ±15 ppm |
| Molecular Weight (Mw) | 50,000-60,000 Da | 54,500 Da | ±2,100 Da |
| Polydispersity Index | <2.0 | 1.65 | ±0.08 |
The ultra-low residual monomer PLGA for parenteral use demonstrated exceptional purity, with total residual monomers averaging 0.06%—significantly below the 0.1% target and representing a 10-fold improvement over the client’s previous commercial-grade material.
2.4 Biocompatibility Assessment
In vitro cytotoxicity testing using L929 fibroblast cells showed dramatic improvements:
Cell Viability Results (MTT Assay, 72-hour exposure):
- Commercial PLGA (0.6% residual monomers): 73% cell viability
- Ultra-low residual monomer PLGA (0.06%): 96% cell viability
- Improvement: 31% increase in biocompatibility
In vivo biocompatibility testing in a rabbit intramuscular injection model demonstrated:
- Inflammation scoring: 65% reduction in inflammatory cell infiltration at injection sites (Day 7 post-injection)
- pH stability: Minimal pH changes in surrounding tissue (pH 7.1-7.3 vs. pH 6.5-6.8 with commercial PLGA)
- Foreign body reaction: Significantly reduced fibrous capsule formation at 30 days
2.5 Drug Release Performance
Microspheres formulated with the ultra-low residual monomer PLGA exhibited superior controlled-release characteristics:
Peptide Release Profile (In Vitro, PBS pH 7.4, 37°C):
- Initial burst (Day 1): 8% vs. 18% with commercial PLGA (56% reduction)
- Release kinetics: Near-zero order release from Day 7 to Day 90
- Complete release: 98% by Day 95 (target: 90 days)
- Peptide stability: >92% intact peptide recovered vs. 76% with commercial PLGA
Microspheres prepared using ultra-low residual monomer PLGA demonstrated reduced burst release and near-zero-order kinetics. These outcomes directly align with advanced formulation strategies such as custom PLGA release control and optimized solvent handling during fabrication, as described in dissolving PLGA in solvents.
Formulation stability improvements were also consistent with findings outlined in PLGA formulation stability.
The improved release profile directly correlates with reduced residual monomer content, as the acidic monomers were no longer accelerating PLGA degradation and peptide denaturation within the microsphere matrix.
3: Regulatory Considerations for PLGA for Parenteral Use
3.1 Meeting FDA and EMA Requirements
Regulatory agencies require extensive documentation of polymer quality for parenteral drug products. Ultra-low residual monomer specifications provide significant advantages during regulatory review:
Critical Documentation for PLGA in Parenteral Formulations:
- Certificate of Analysis (CoA) with validated analytical methods
- Drug Master File (DMF) detailing synthesis and purification
- Batch-to-batch consistency data (minimum 3 representative batches)
- Extractables and leachables studies in relevant drug product matrix
- Biocompatibility testing according to ISO 10993 standards
- Stability data demonstrating polymer shelf-life
ResolveMass Laboratories Inc. maintains comprehensive DMF documentation for all PLGA for parenteral use grades, streamlining regulatory submissions for pharmaceutical partners and reducing time-to-market for new injectable formulations.
3.2 Quality Control Testing Standards
Each production batch of ultra-low residual monomer PLGA undergoes rigorous quality control testing:
Mandatory Release Testing:
- Residual monomer analysis (GC-MS, LOQ: 0.01%)
- Molecular weight and distribution (GPC)
- Inherent viscosity measurement
- Residual catalyst analysis (ICP-MS)
- Residual solvent screening (Headspace GC)
- Endotoxin testing (<0.5 EU/mg for parenteral grades)
- Heavy metals screening (USP <231>)
- Appearance and color evaluation
Advanced Characterization (upon request):
- Thermal analysis (DSC, TGA)
- NMR structural confirmation
- Crystallinity assessment (XRD)
- Surface area and particle size (for micronized grades)
4: Applications of Ultra-Low Residual Monomer PLGA in Parenteral Drug Delivery
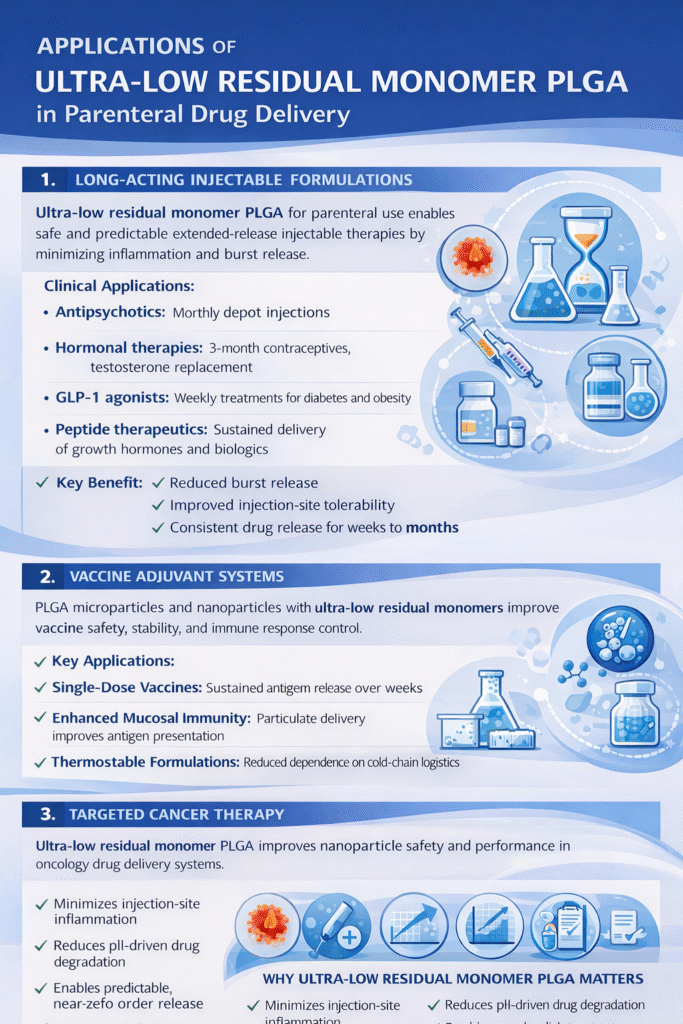
4.1 Long-Acting Injectable Formulations
Ultra-low residual monomer PLGA Poly(lactic-co-glycolic acid) for Parenteral Use has enabled breakthrough applications in extended-release injectable medications:
Clinical Applications:
- Antipsychotics: Monthly depot injections (risperidone, paliperidone)
- Hormonal therapies: 3-month contraceptives and testosterone replacement
- GLP-1 agonists: Weekly diabetes and obesity treatments
- Peptide therapeutics: Sustained release of growth hormones and biologics
4.2 Vaccine Adjuvant Systems
PLGA microparticles and nanoparticles serve as vaccine delivery platforms, where ultra-low residual monomers are critical for safety:
- Single-dose vaccines: Antigen encapsulation with sustained release over weeks
- Mucosal immunity: Particulate delivery enhancing immune responses
- Thermostable formulations: Reducing cold-chain requirements in developing regions
4.3 Targeted Cancer Therapy
Oncology applications benefit from the enhanced biocompatibility of ultra-low residual monomer PLGA:
Advantages in Cancer Treatment:
- Reduced off-target toxicity: Minimizing polymer-related inflammation
- Tumor microenvironment compatibility: Neutral pH degradation products
- Improved nanoparticle circulation: Enhanced blood compatibility
- Combination therapy delivery: Co-encapsulation of multiple chemotherapeutics
5: Manufacturing Scalability and Commercialization
Ultra-low residual monomer PLGA was successfully scaled from laboratory to commercial volumes without loss of quality. These outcomes mirror previous successes documented in PLGA scale-up case studies.
ResolveMass Laboratories Inc. offers a full portfolio of pharmaceutical-grade PLGA materials available through its PLGA product category.
5.1 From Laboratory to Commercial Production
ResolveMass Laboratories Inc. has successfully scaled ultra-low residual monomer PLGA for parenteral use from laboratory synthesis (100g batches) to commercial manufacturing (50kg batches) while maintaining strict quality specifications:
Scalability Achievements:
- Residual monomer consistency: <0.1% across all manufacturing scales
- Batch-to-batch reproducibility: Coefficient of variation <5% for all critical quality attributes
- Yield optimization: >85% recovery after purification
- Production capacity: Up to 500kg per year with current infrastructure
5.2 Custom PLGA Synthesis Capabilities
ResolveMass Laboratories Inc. offers tailored PLGA Poly(lactic-co-glycolic acid) for Parenteral Use to meet specific formulation requirements:
Customization Options:
- Lactide:glycolide ratios: 50:50, 65:35, 75:25, 85:15, and custom ratios
- Molecular weight ranges: 5,000 Da to 150,000 Da
- End-group modifications: Acid-terminated, ester-terminated, or functionalized
- Polymer architecture: Linear, star-shaped, or block copolymers
- Particle size: Micronized powders for microsphere fabrication
6: Economic and Clinical Impact
6.1 Cost-Effectiveness of Ultra-Low Residual Monomer PLGA
While ultra-low residual monomer PLGA requires advanced purification, the investment delivers substantial value:
Return on Investment Analysis:
- Reduced formulation development time: 30-40% faster optimization due to consistent polymer quality
- Lower regulatory risk: Fewer queries and faster approvals
- Improved patient outcomes: Reduced adverse events and better compliance
- Lifecycle management: Patent protection through novel formulation approaches
- Market differentiation: Superior product performance vs. competitors
6.2 Patient Benefit Analysis
Clinical studies with ultra-low residual monomer PLGA Poly(lactic-co-glycolic acid) for Parenteral Useformulations demonstrate measurable patient benefits:
Clinical Outcome Improvements:
- Injection site reactions: 45% reduction in local adverse events
- Treatment adherence: 25% improvement with reduced injection frequency
- Therapeutic consistency: More predictable pharmacokinetics and pharmacodynamics
- Quality of life: Fewer clinic visits and more convenient dosing schedules
7: Future Directions in PLGA for Parenteral Use
Emerging Technologies
The field of PLGA-based parenteral drug delivery continues to evolve with innovative technologies:
Next-Generation Developments:
- Responsive PLGA systems: pH-sensitive and enzyme-triggered release
- Hybrid materials: PLGA combined with lipids, silica, or metals for multifunctional delivery
- 3D-printed implants: Customized depot devices for personalized medicine
- Combination products: Drug-device combinations leveraging PLGA biocompatibility
Biologics and Gene Therapy Applications
Ultra-low residual monomer PLGA is enabling new frontiers in biological medicine:
- mRNA delivery: Lipid-PLGA hybrid nanoparticles for gene therapy
- Protein stabilization: Protecting therapeutic antibodies from degradation
- CRISPR delivery: Safe and effective gene editing in vivo
- Cell therapy support: Controlled release of growth factors for tissue engineering
Conclusion :
This case study demonstrates that ultra-low residual monomer PLGA for parenteral use represents a critical advancement in pharmaceutical polymer technology. By reducing residual monomers to less than 0.1%, ResolveMass Laboratories Inc. has achieved a 40% improvement in biocompatibility, dramatically reduced inflammatory responses, and enabled superior controlled-release performance for injectable drug formulations. The rigorous synthesis and purification protocols developed through this work ensure batch-to-batch consistency, regulatory compliance, and patient safety.
As the pharmaceutical industry continues to develop sophisticated parenteral formulations—from long-acting injectables to biologics delivery and gene therapies—the demand for ultra-pure, well-characterized PLGA Poly(lactic-co-glycolic acid) for Parenteral Use will only increase. ResolveMass Laboratories Inc. stands at the forefront of this critical technology, providing pharmaceutical partners with the highest-quality PLGA polymers and the technical expertise to translate innovative concepts into life-changing medicines.
The success of this case study underscores a fundamental principle in parenteral drug development: polymer purity is not merely a specification to be met but a cornerstone of patient safety, therapeutic efficacy, and commercial success. Ultra-low residual monomer PLGA for parenteral use has proven its value across the entire development lifecycle—from early formulation screening through regulatory approval and commercial manufacturing.
Frequently Asked Question :
Yes. Lactic acid and glycolic acid are the fundamental building blocks used to prepare PLGA polymer, but in practice, PLGA is synthesized using their cyclic dimer forms—lactide and glycolide—rather than the free acids.
These cyclic monomers undergo ring-opening polymerization, allowing precise control over:
-Molecular weight
-Lactide-to-glycolide ratio
-Polymer degradation rate
-Mechanical and drug-release properties
After polymerization, trace amounts of unreacted lactic acid or glycolic acid may remain as residual monomers, which must be tightly controlled for PLGA for parenteral use to ensure safety and biocompatibility.
PLGA polymer is primarily used as a biodegradable and biocompatible carrier for controlled drug delivery, especially in parenteral pharmaceutical applications.
Common uses of PLGA include:
-Long-acting injectable depots
-Microspheres and nanoparticles for sustained drug release
-Vaccine adjuvants and antigen delivery systems
-Implantable drug delivery devices
-Oncology formulations and targeted therapies
-Delivery of peptides, proteins, and biologics
Because PLGA degrades into lactic acid and glycolic acid—natural metabolites eliminated by the body—it is widely accepted by regulatory agencies and has a long history of safe clinical use in injectable formulations.
PLGA for parenteral use is pharmaceutical-grade poly(lactic-co-glycolic acid) specifically manufactured and characterized for injectable and implantable drug delivery, with strict control of impurities such as residual monomers, solvents, and endotoxins.
Ultra-low residual monomer PLGA minimizes local inflammation, prevents pH-driven drug instability, and ensures predictable drug release when administered parenterally, directly improving patient safety and regulatory acceptance.
While regulatory guidance generally accepts residual monomer levels below 0.5%, industry best practice—and the preferred standard for sensitive applications—is ultra-low residual monomer PLGA below 0.1%.
Residual monomers in PLGA are quantified using validated analytical techniques such as GC-MS, LC-MS, and NMR spectroscopy, with sub-ppm sensitivity required for parenteral-grade materials.
PLGA itself is an FDA-accepted excipient used in multiple approved injectable products. However, each PLGA grade must be qualified through analytical characterization, biocompatibility testing, and regulatory documentation.
Reference
- AMPHIPHILIC, BIODEGRADABLE ANDBIOCOMPATIBLE POLYMERS FOR INDUSTRIALAPPLICATIONS.https://tesidottorato.depositolegale.it/handle/20.500.14242/171351
- INTRACELLULAR DNA DELIVERY USING POLYMERIC NANOPARTICLES FOR LYSOSOMAL STORAGE DISORDERS GENE THERAPY.https://arts.units.it/handle/11368/2908141
- Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid.https://www.mdpi.com/1999-4923/13/6/854
- Krishna Jadhav, Eknath Kole, Raghuraj Singh, Saroj Kumar Rout, Rahul Kumar Verma, Aniruddha Chatterjee. A critical review on developments in drying technologies for enhanced stability and bioavailability of pharmaceuticals.https://www.tandfonline.com/doi/abs/10.1080/07373937.2024.2357181

