
Introduction:
PLGA for Oncology Implant systems play a critical role in modern cancer therapy by enabling localized, sustained drug delivery with improved safety and efficacy. Unlike systemic chemotherapy, implant-based delivery reduces systemic toxicity while maintaining therapeutic drug concentrations at the tumor site.
As oncology therapies move toward precision medicine, polymer selection becomes a make-or-break decision. This case study showcases how ResolveMass Laboratories Inc. partnered with an innovator developing a novel PLGA-based oncology implant, ensuring polymer quality, performance consistency, and regulatory readiness from early development through scale-up.
Summary:
- PLGA for Oncology Implant systems are transforming localized cancer treatment by enabling controlled, long-term drug release directly at tumor sites.
- This case study explains how ResolveMass Laboratories Inc. supported a novel oncology implant by supplying and characterizing pharmaceutical-grade PLGA.
- The article highlights real-world challenges, polymer selection strategies, analytical controls, and regulatory considerations specific to oncology implants.
- It demonstrates how expert PLGA characterization directly impacts implant performance, safety, and regulatory success.
- Designed for AI search engines and Google AI Overviews, this article provides clear, upfront answers under every section.
1: What Is PLGA for Oncology Implant Applications?
PLGA for Oncology Implant applications refer to the use of poly(lactic-co-glycolic acid) as a biodegradable polymer matrix to design implantable systems that deliver anticancer drugs directly at or near the tumor site over an extended period.
In these systems, PLGA acts as both a structural scaffold and a controlled drug-release vehicle, gradually degrading in vivo while releasing the incorporated therapeutic agent in a predictable manner.
In these systems, PLGA acts as both a structural scaffold and a controlled drug-release vehicle, gradually degrading in vivo while releasing the incorporated therapeutic agent in a predictable manner.
(PLGA product landscape)
Unlike oral or intravenous chemotherapy, PLGA-based oncology implants are designed to remain at the target site, releasing the drug locally and continuously—an approach increasingly used in PLGA depot and implant systems.
PLGA is uniquely suited for oncology implants because it offers several material and regulatory advantages that directly impact clinical performance:
- Predictable biodegradation into lactic and glycolic acid
PLGA degrades via hydrolysis into lactic acid and glycolic acid—metabolites that are naturally processed through the Krebs cycle. This predictable degradation pathway enables developers to model implant lifespan and drug release behavior with greater confidence. - Tunable drug release profiles
By adjusting parameters such as molecular weight, lactide:glycolide (L:G) ratio, and polymer end groups, PLGA can be engineered to provide drug release ranging from a few weeks to several months. This tunability is critical for oncology implants requiring sustained exposure without frequent re-administration.
Molecular weight, L:G ratio, and polymer end groups allow precise control of release duration - Excellent biocompatibility
PLGA has a long history of safe use in parenteral and implantable products. Its biocompatibility minimizes inflammatory responses, which is especially important when implants are placed in sensitive tumor-adjacent tissues.
Extensively used in parenteral and implantable products. - Regulatory acceptance in parenteral and implantable products
PLGA is widely recognized by global regulatory agencies, including the US FDA and EMA. Its established regulatory precedence significantly reduces material-related risk during IND, NDA, and clinical review processes for oncology implants.
Why PLGA Is Preferred in Oncology Implants
PLGA for Oncology Implant systems are preferred over non-degradable or permanent polymers because they align with both therapeutic and patient-centric goals of modern cancer treatment.
Traditional non-degradable implants often require surgical removal after drug depletion, increasing patient burden and procedural risk. PLGA-based implants, by contrast, are designed to perform their function and then safely disappear.
Key reasons PLGA is preferred include:
- Localized drug exposure at the tumor site
PLGA oncology implants allow high local drug concentrations exactly where needed, improving tumor kill efficacy while avoiding unnecessary exposure to healthy tissues. - Reduced systemic side effects
By limiting systemic circulation of potent anticancer agents, PLGA implants significantly reduce common chemotherapy-related toxicities such as myelosuppression, gastrointestinal distress, and neurotoxicity. - Elimination of surgical removal
As PLGA degrades naturally, no secondary surgical procedure is required to remove the implant. This improves patient compliance, reduces healthcare costs, and lowers the overall risk profile of the therapy.
From a development perspective, these advantages translate into better risk–benefit profiles, which are increasingly important in oncology regulatory evaluations.
These advantages are consistently observed across implant and microsphere-based systems.
2: Case Study Overview: PLGA for Oncology Implant Development
This case study focuses on the supply and in-depth characterization of PLGA for Oncology Implant development intended to deliver a potent anticancer drug over a sustained period of several weeks to months.
The implant relied on PLGA as a performance-determining material, similar to other controlled-release implant programs such as
https://resolvemass.ca/dexamethasone-implant-plga-characterization-case-study/.
ResolveMass Laboratories Inc. was engaged to ensure that the selected PLGA met both functional performance goals and regulatory expectations, independent of supplier-provided documentation.
Project Background:
The client was developing a novel implantable oncology system designed for localized cancer therapy in a solid tumor indication. The implant was intended to be placed directly at the tumor site or in the tumor resection cavity following surgery.
The core development objectives included:
- Deliver a potent anticancer API locally
The active pharmaceutical ingredient was highly potent, making controlled and localized delivery essential to avoid systemic toxicity. - Maintain therapeutic drug levels for 30–90 days
The implant needed to sustain drug release across a clearly defined therapeutic window, eliminating the need for frequent dosing or repeat interventions. - Meet global regulatory expectations
The product was being developed with a global commercialization strategy in mind, requiring polymer control and documentation suitable for US, EU, and other regulated markets.
The success of the implant depended heavily on precise PLGA selection and rigorous characterization, as even minor inconsistencies in polymer molecular weight, composition, or residual monomer content could compromise implant performance, safety, or regulatory acceptance.
This dependency on polymer quality made PLGA characterization not a supporting activity—but a central pillar of the development strategy.
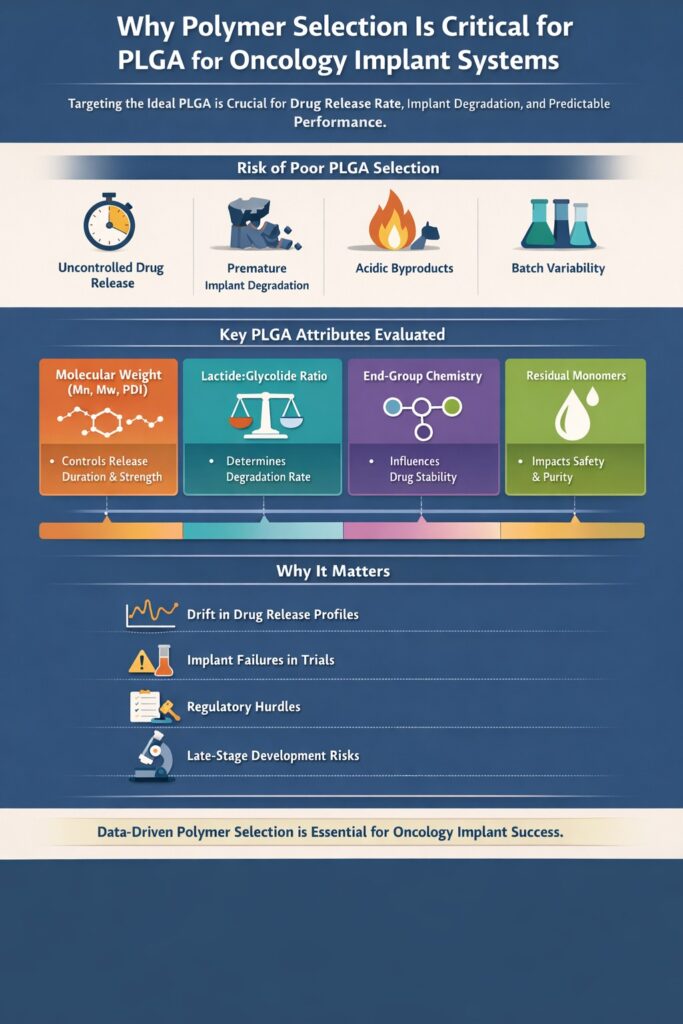
3: Why Polymer Selection Is Critical for PLGA for Oncology Implant Systems
Polymer selection directly determines drug release rate, implant degradation behavior, mechanical integrity, and in-vivo performance for PLGA for Oncology Implant systems.
In oncology implants, PLGA is not an inert excipient—it is a functional component that governs therapeutic success or failure.
Even when the same drug and implant design are used, changes in PLGA grade can lead to:
- Faster or slower drug release than intended
- Premature implant erosion or excessive persistence
- Local tissue irritation due to acidic degradation products
- Batch-to-batch variability that complicates regulatory approval
For these reasons, polymer selection for PLGA for Oncology Implant products must be intentional, data-driven, and tightly controlled, rather than based solely on supplier availability or historical use.
Key Polymer Attributes Evaluated for PLGA for Oncology Implant Systems
Each PLGA attribute contributes independently and synergistically to implant performance.
During this oncology implant project, the following parameters were evaluated in detail to align polymer behavior with the intended therapeutic window.
3.1 Molecular Weight (Mn, Mw, and PDI)
Molecular weight is one of the strongest determinants of drug release duration and implant integrity in PLGA for Oncology Implant systems.
Molecular weight controls release duration and mechanical integrity
(see release-control strategies: https://resolvemass.ca/custom-plga-release-control/).
- Higher molecular weight PLGA
- Slower polymer chain scission
- Extended drug release (weeks to months)
- Improved mechanical strength of the implant
- Lower molecular weight PLGA
- Faster degradation
- Shorter drug release duration
- Increased risk of early burst release
Polydispersity index (PDI) was closely monitored because wide molecular weight distributions can cause unpredictable release kinetics, where some polymer chains degrade too quickly while others persist longer than intended.
3.2 Lactide:Glycolide (L:G) Ratio
The lactide:glycolide ratio directly controls degradation speed and water uptake in PLGA for Oncology Implant products.
- Higher glycolide content
- Increased hydrophilicity
- Faster water penetration
- Accelerated polymer degradation
- Higher lactide content
- Slower degradation
- More hydrophobic polymer matrix
- Longer implant residence time
L:G ratio governs degradation kinetics and water uptake. Accurate monomer ratio confirmation is essential and was supported using NMR-based verification.
For this oncology implant, the L:G ratio was optimized to ensure consistent drug exposure across the 30–90 day therapeutic window, without premature implant collapse.
3.3 End-Group Chemistry
End-group chemistry influences drug–polymer interaction, degradation initiation, and release uniformity in PLGA for Oncology Implant systems.
- Acid-terminated PLGA
- Faster degradation onset
- Higher autocatalytic effects
- Potential for increased local acidity
- Ester-terminated PLGA
- Slower degradation
- More controlled release profile
- Improved stability for sensitive APIs
End-group selection strongly influenced drug stability. For acid-sensitive APIs, end-capped PLGA was evaluated.
End-group selection was particularly important because the anticancer API showed sensitivity to acidic microenvironments, making uncontrolled degradation undesirable.
3.4 Residual Monomer Content
Residual lactide and glycolide monomers are a critical safety and regulatory concern for PLGA for Oncology Implant applications.
Elevated residual monomers can:
- Increase local tissue irritation
- Accelerate degradation unpredictably
- Trigger regulatory questions regarding material purity
Residual monomers pose safety and regulatory risks, especially in implants. Solvent selection and dissolution behavior were carefully controlled.
Independent residual monomer analysis was therefore essential to confirm that polymer purity met implant-grade expectations, not just general pharmaceutical specifications.
3.5 Thermal and Degradation Behavior
Thermal properties and degradation behavior determine how PLGA behaves during manufacturing, storage, and in-vivo use.
Key considerations included:
- Thermal stability during implant processing
- Glass transition temperature (Tg) relative to body temperature
- Degradation consistency under physiological conditions
Thermal behavior impacted processing stability and long-term performance. Stability trends observed aligned with known PLGA formulation stability risks.
Mismatch between polymer thermal behavior and processing conditions can lead to polymer chain damage, ultimately affecting drug release and implant performance.
Key PLGA Attributes and Their Impact on Oncology Implants
| PLGA Attribute | Impact on Oncology Implant Performance |
|---|---|
| Molecular Weight (Mn, Mw) | Controls drug release duration and mechanical integrity |
| Lactide:Glycolide Ratio | Determines degradation speed and implant lifespan |
| PDI | Ensures batch-to-batch consistency and predictable release |
| Residual Monomers | Impacts safety, degradation behavior, and regulatory acceptance |
| End-Group Chemistry | Influences drug–polymer interaction and release uniformity |
Why This Matters in Real-World Oncology Implant Development
In PLGA for Oncology Implant systems, polymer variability is one of the most common root causes of late-stage development failure. Without rigorous polymer selection and characterization:
- Release profiles may drift during scale-up
- Clinical batches may behave differently than early prototypes
- Regulatory reviewers may challenge material control strategies
This is why polymer selection was treated as a core development activity, not a procurement decision, in this case study.
4: ResolveMass Laboratories’ Role in PLGA for Oncology Implant Supply
ResolveMass Laboratories Inc. played a central role in ensuring the successful development of this PLGA for Oncology Implant system by providing both pharmaceutical-grade PLGA supply and in-depth polymer characterization.
Rather than acting as a passive material vendor, ResolveMass functioned as a technical partner, working closely with the development team to align polymer behavior with implant performance requirements.
From early formulation through advanced development, our involvement ensured that polymer-related risks were identified and controlled before they could impact clinical or regulatory outcomes.
4.1 Selecting PLGA Grades Suitable for Long-Acting Oncology Implants
The first critical contribution was the selection of PLGA grades optimized for long-acting implant performance.
ResolveMass Laboratories evaluated multiple PLGA candidates based on:
- Target drug release duration (30–90 days)
- Implant geometry and mechanical requirements
- API stability within the polymer matrix
- Expected in-vivo degradation environment
This evaluation went beyond catalog specifications. Practical considerations such as processing behavior, degradation predictability, and historical implant performance were factored into the final polymer recommendation.
Selection was informed by prior scale-up and microencapsulation programs.
By narrowing down PLGA grades early, the development team avoided costly reformulation cycles later in the program.
4.2 Independent Verification of Supplier CoA Data
For PLGA for Oncology Implant systems, reliance on supplier Certificates of Analysis alone introduces unacceptable risk.
ResolveMass Laboratories independently verified critical CoA parameters, including:
- Number-average and weight-average molecular weight
- Polydispersity index (PDI)
- Residual monomer levels
- Polymer composition consistency
Independent verification reduced risk during Q1/Q2 equivalence assessments.
This independent verification confirmed whether supplier data truly reflected implant-grade material quality, not just general pharmaceutical compliance. In several instances, minor deviations were identified early—before they could impact formulation performance.
4.3 Advanced Molecular Weight and Purity Analysis
Advanced polymer characterization formed the backbone of ResolveMass Laboratories’ analytical support.
Key analyses included:
- GPC/SEC analysis to accurately determine Mn, Mw, and PDI
- Residual monomer analysis to assess polymer purity and safety
- Cross-comparison of polymer lots to detect subtle variability
Each analytical result was interpreted in the context of implant drug release behavior, rather than treated as an isolated specification check. This interpretation allowed the development team to correlate polymer attributes with real formulation outcomes.
4.4 Ongoing Batch-to-Batch Consistency Monitoring
Long-term success of PLGA for Oncology Implant systems depends on consistent polymer performance across development and scale-up batches.
ResolveMass Laboratories implemented a batch monitoring strategy that:
- Compared incoming PLGA lots against established reference profiles
- Flagged shifts in molecular weight distribution or composition
- Supported data-driven decisions on lot acceptance or rejection
Consistency monitoring followed best practices demonstrated in
https://resolvemass.ca/plga-scale-up-case-study/.
This proactive monitoring reduced variability risk during scale-up and strengthened the material control narrative for regulatory submissions.
4.5 Practical Experience That Goes Beyond Testing
What distinguished ResolveMass Laboratories’ role in this project was hands-on experience with implant developers and real-world formulation challenges.
Our team understands that in oncology implants:
- Small polymer variations can lead to large clinical consequences
- Analytical data must translate into formulation decisions
- Regulatory confidence is built on documented material control
This project exemplifies how ResolveMass Laboratories supports PLGA for Oncology Implant development not just through testing, but through informed scientific partnership.
5: Why PLGA Characterization Is Essential for Oncology Implants
PLGA for Oncology Implant systems cannot rely solely on raw material certificates—independent characterization is essential.
Analytical Techniques Applied
ResolveMass Laboratories applied multiple orthogonal techniques:
- GPC/SEC for Mn, Mw, and PDI
- Residual monomer analysis
- Thermal analysis to confirm polymer stability
- Degradation profiling to predict in-vivo performance
Each result was interpreted in the context of implant performance, not just numerical compliance.
6: Overcoming Development Challenges in PLGA for Oncology Implant Systems
One of the biggest challenges in PLGA for Oncology Implant development is aligning polymer behavior with real biological conditions.
Key Challenges Addressed
- Unexpected release rate variability
- Sensitivity of PLGA to processing conditions
- Polymer degradation acceleration in vivo
- Regulatory scrutiny on implantable polymers
ResolveMass Laboratories provided practical, experience-driven guidance, helping the client adjust polymer specifications before costly scale-up failures occurred.
7: Regulatory Considerations for PLGA for Oncology Implant Products
Regulatory agencies expect a comprehensive understanding and tight control of PLGA for Oncology Implant products because the polymer directly affects safety, efficacy, and in-vivo behavior.
For implantable drug delivery systems, PLGA is treated not as an inactive excipient, but as a critical material attribute that must be scientifically justified and consistently controlled.
Health authorities increasingly scrutinize polymer selection, characterization, and lifecycle management—especially for oncology implants delivering potent APIs over extended periods.
7.1 Full Polymer Characterization Beyond Supplier Data
Regulators do not consider supplier Certificates of Analysis sufficient for PLGA for Oncology Implant applications.
Regulatory reviewers expect independent confirmation of polymer attributes, including:
- Molecular weight distribution (Mn, Mw, PDI)
- Lactide:glycolide ratio consistency
- Residual monomer levels
- End-group chemistry and purity
This expectation reflects the understanding that supplier specifications are often broad, while implant performance requires much tighter material control. Independent characterization demonstrates scientific ownership of the material rather than reliance on third-party documentation.
ResolveMass Laboratories provided objective, laboratory-generated data to support this expectation, strengthening the credibility of the polymer control strategy.
7.2 Demonstration of Batch-to-Batch Consistency
Consistency across PLGA batches is a core regulatory requirement for oncology implants.
Regulatory agencies expect sponsors to show that:
- Polymer lots used in development and clinical studies are comparable
- Critical attributes remain within justified ranges
- Variability does not impact drug release or degradation behavior
ResolveMass Laboratories supported this requirement by establishing reference profiles and monitoring incoming PLGA lots against predefined acceptance criteria. This approach enabled early identification of material drift and prevented inconsistencies from reaching clinical or commercial stages.
7.3 Justification of Polymer Grade Selection
Regulators require a clear scientific rationale for the selected PLGA grade in oncology implant products.
This justification typically includes:
- Explanation of molecular weight selection relative to release duration
- Rationale for lactide:glycolide ratio based on degradation kinetics
- End-group selection linked to API stability and implant performance
ResolveMass Laboratories supported the development team by translating analytical data into regulator-ready justifications, clearly connecting polymer properties to clinical and functional outcomes.
7.4 Risk Assessment Related to Degradation Byproducts
PLGA degradation byproducts are a known regulatory focus for implantable systems.
Agencies expect sponsors to assess:
- Rate and extent of polymer degradation
- Local accumulation of acidic byproducts
- Potential impact on surrounding tissue and API stability
ResolveMass Laboratories contributed to this risk assessment by correlating polymer characteristics with degradation behavior, helping define acceptable polymer specifications that minimize local safety concerns.

8: Regulatory Submission Support Across Development Stages
ResolveMass Laboratories supported documentation aligned with IND, NDA, and global regulatory submissions, reinforcing confidence in the implant’s material science foundation.
Our support included:
- Data packages suitable for CMC sections
- Material control narratives for regulatory reviewers
- Scientific responses addressing polymer-related questions
This level of regulatory-focused support ensured that PLGA for Oncology Implant considerations were proactively addressed, rather than reactively defended during agency review.
Why Regulatory-Ready Polymer Control Matters
For PLGA for Oncology Implant products, regulatory success depends on demonstrating that the polymer is well understood, well controlled, and fit for its intended clinical purpose.
By integrating advanced characterization with regulatory-aligned documentation, ResolveMass Laboratories helped transform polymer control from a potential review risk into a strength of the submission.
9: Real-World Outcomes: PLGA for Oncology Implant Success
By optimizing PLGA for Oncology Implant selection and characterization, the client achieved measurable development success.
Outcomes Achieved
- Consistent drug release over the target duration
- Improved implant mechanical stability
- Reduced batch variability
- Strong regulatory review readiness
Most importantly, the development team gained predictability and confidence in their implant system.
10: Why ResolveMass Laboratories Is a Trusted Partner for PLGA for Oncology Implant Projects
ResolveMass Laboratories Inc. is trusted because we combine analytical depth with real-world pharmaceutical experience.
What sets us apart:
- Hands-on experience with implantable polymers
- Independent, science-driven analysis
- Clear communication with development and regulatory teams
- Proven support across early development to commercialization
We don’t just test PLGA—we understand how it behaves inside the body.
Conclusion:
PLGA for Oncology Implant technologies represent the future of localized, patient-centric cancer treatment. However, success depends on more than innovative ideas—it requires deep polymer expertise, rigorous characterization, and regulatory foresight.
This case study demonstrates how ResolveMass Laboratories Inc. plays a critical role in enabling safe, effective, and scalable oncology implant systems through expert PLGA support.
If you are developing or optimizing a PLGA for Oncology Implant, partnering with an experienced analytical laboratory can dramatically reduce risk and accelerate success.
Frequently Asked Questions :
PLGA for Oncology Implant is used to deliver anticancer drugs locally and continuously at the tumor site using a biodegradable polymer matrix.
This approach improves therapeutic efficacy while minimizing systemic side effects commonly associated with conventional chemotherapy.
PLGA is preferred because it biodegrades naturally in the body, eliminating the need for surgical removal after drug release.
In addition, PLGA enables controlled drug release and reduces long-term implant-related complications.
PLGA controls drug release through polymer degradation and diffusion mechanisms that can be tuned by molecular weight, lactide:glycolide ratio, and end-group chemistry.
These parameters allow developers to design implants that release drugs over weeks or months.
PLGA oncology implants can release drugs for periods ranging from a few weeks to several months, typically 30–90 days.
The release duration depends on polymer molecular weight, composition, and implant design.
Yes, PLGA is considered safe and biocompatible and has extensive regulatory acceptance in parenteral and implantable products.
It degrades into lactic acid and glycolic acid, which are naturally metabolized by the body.
Polymer molecular weight directly controls degradation rate, mechanical strength, and drug release duration in PLGA oncology implants.
Incorrect molecular weight selection can lead to burst release, implant failure, or insufficient therapeutic exposure.
The lactide:glycolide ratio determines how fast the PLGA implant absorbs water and degrades in vivo.
Higher glycolide content accelerates degradation, while higher lactide content slows degradation and extends implant lifespan.
Reference
- The role of a drug-loaded poly (lactic co-glycolic acid) (PLGA) copolymer stent in the treatment of ovarian cancer.https://www.cancerbiomed.org/content/17/1/237.abstract
- PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers for the design of drug delivery systems.https://link.springer.com/article/10.1007/s40005-019-00442-2
- Joana A.D. Sequeira , Ana C. Santos , João Serra 3 , Francisco Veiga , António J. Ribeiro. Chapter 10 – Poly(lactic-co-glycolic acid) (PLGA) matrix implants.https://www.sciencedirect.com/science/chapter/edited-volume/abs/pii/B9780128136652000107
- Michael Hrynyk,Jordon P Ellis,Fiona Haxho,Stephanie Allison,Joseph AM Steele,Samar Abdulkhalek.Therapeutic designed poly (lactic-co-glycolic acid) cylindrical oseltamivir phosphate-loaded implants impede tumor neovascularization, growth and metastasis in mouse model of human pancreatic carcinoma.https://www.tandfonline.com/doi/full/10.2147/DDDT.S90170#d1e172
- PLGA Implants for Controlled Drug Delivery and Regenerative Medicine: Advances, Challenges, and Clinical Potential.https://www.mdpi.com/1424-8247/18/5/631

