Introduction:
IND enabling Bioanalytical Studies are the scientific proof that a drug can be safely tested in humans.
Before a new drug can enter human testing, regulators must understand not just what dose was given, but how much drug actually reached the body and produced an effect. That evidence comes from IND enabling Bioanalytical Studies, which translate animal study outcomes into scientifically justified clinical dosing strategies.
Across many development programs, it is often not toxicity itself that delays an IND — it is the inability to connect observed effects with reliable exposure data. When concentrations, metabolites, and biological responses are clearly characterized, regulators can confidently evaluate safety margins and allow clinical trials to proceed.
In short, bioanalysis converts preclinical results into regulatory confidence.
Learn more about why bioanalysis matters
Summary:
- IND enabling Bioanalytical Studies provide the exposure data regulators require before human trials can begin.
- They connect toxicology findings to actual drug concentrations in the body.
- Pharmacokinetics (PK) and toxicokinetics (TK) determine starting dose and safety margin.
- Method validation is legally required — unvalidated assays invalidate GLP studies.
- Metabolite monitoring prevents late-stage clinical failure.
- Biomarkers strengthen mechanistic justification and reduce regulatory questions.
- Early bioanalytical planning can prevent clinical holds and save millions in repeat studies.
1: What Are IND Enabling Bioanalytical Studies?
IND enabling Bioanalytical Studies quantify drug, metabolite, and biomarker levels in biological matrices to interpret safety and support human dosing decisions.
They are conducted during nonclinical pharmacology and toxicology phases and form a mandatory component of regulatory submission.
Biological Matrices Typically Analyzed
- Plasma or serum
- Whole blood
- Urine and feces
- Tissue homogenates
- Cerebrospinal fluid
- Ocular fluid
- Tumor tissue
- Synovial fluid
Related services:
https://resolvemass.ca/bioanalytical-services/
https://resolvemass.ca/bioanalytical-quantification/
https://resolvemass.ca/lc-ms-ms-bioanalytical-services/
Biological Matrices
Plasma, blood, urine, tissue, CSF, ocular fluid, tumor samples
Matrix challenges explained.
2: Why IND Enabling Bioanalytical Studies Are Required
Regulators must understand exposure — not just administered dose — to determine risk.
Two animals given the same dose may experience different toxicity because their systemic exposure differs. Bioanalysis resolves this uncertainty.
Regulatory Decisions Based on Bioanalytical Data
- Human starting dose
- Dose escalation scheme
- Maximum clinical exposure
- Safety margins
- Accumulation risk
- Species relevance
Related regulatory support:
Bioanalytical Services for IND-NDA Submission.
Regulated Bioanalytical Services
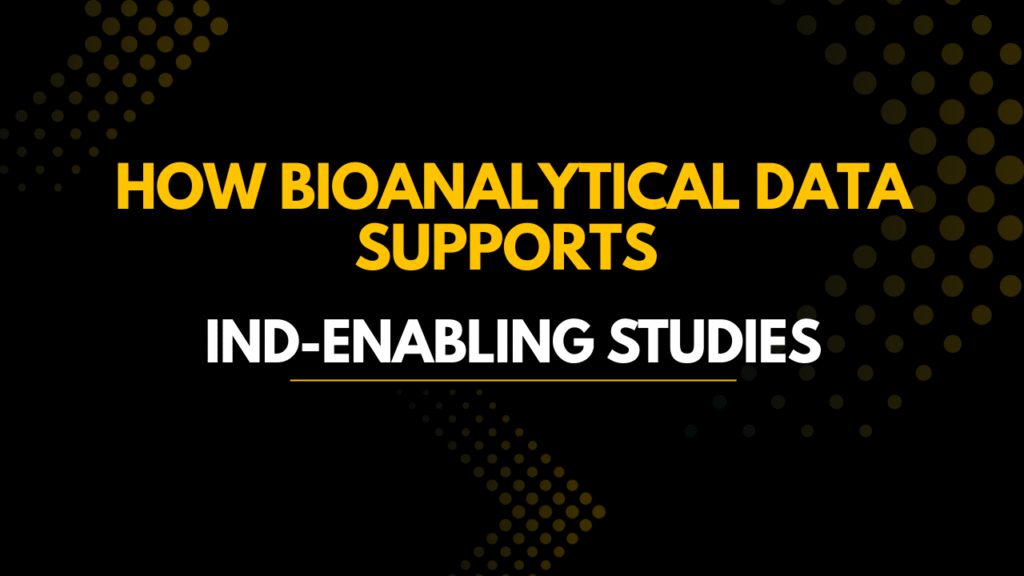
3: Core Components of IND Enabling Bioanalytical Studies
1. Pharmacokinetics (PK)
PK determines how the body handles the drug.
It answers:
How fast does drug enter circulation?
How long does it stay?
Does it accumulate?
Key PK Parameters
| Parameter | Meaning | IND Impact |
|---|---|---|
| Cmax | Peak concentration | Acute toxicity risk |
| AUC | Total exposure | Safety margin |
| Tmax | Absorption rate | Dosing schedule |
| Half-life | Elimination speed | Frequency |
| Clearance | Drug removal | Human prediction |
2. Toxicokinetics (TK)
TK is the most critical part of IND enabling Bioanalytical Studies because it links toxicity to exposure.
Toxicology without TK is uninterpretable.
TK Determines
- Exposure at NOAEL
- Dose proportionality
- Gender differences
- Accumulation during repeat dosing
- Target organ exposure
TK bioanalysis:
https://resolvemass.ca/toxicokinetic-bioanalysis/
https://resolvemass.ca/bioanalytical-cro-services-for-pk-and-tk/
3. Bioanalytical Method Validation
Regulators only accept data generated from validated methods.
If a GLP study uses an unvalidated assay, the entire toxicology study may be rejected.
Mandatory Validation Tests
| Test | Purpose |
|---|---|
| Accuracy | True concentration measurement |
| Precision | Repeatability |
| Selectivity | No interference |
| Stability | Sample reliability |
| Matrix effect | Ion suppression detection |
| Recovery | Extraction efficiency |
| Carryover | Contamination control |
Method validation guidance:
Bioanalytical Method Validation
Bioanalytical Method Development
Challenges Bioanalytical Method Development
Rapid Bioanalytical Method Development
Stability considerations:
Bioanalytical Stability Testing
4: How Bioanalytical Data Determines Starting Human Dose
Human starting dose is derived from exposure margin — not from administered animal dose.
Calculation Logic
- Determine NOAEL exposure in animals
- Predict human exposure
- Apply safety factor
- Select starting dose
Example
| Parameter | Value |
|---|---|
| Animal NOAEL AUC | 12,000 ng·h/mL |
| Predicted human AUC | 600 ng·h/mL |
| Safety margin | 20× |
| Result | Acceptable for Phase I |
Advanced modeling strategies:
Virtual Bioanalytical Strategy
Without IND enabling Bioanalytical Studies, this calculation cannot be performed — and the IND will not proceed.
5: Metabolite Monitoring in IND Enabling Bioanalytical Studies
Human-unique or disproportionate metabolites must be evaluated for safety.
Some drugs fail in Phase I not because of parent compound toxicity — but because a metabolite appears only in humans.
Required Assessments
- Cross-species comparison
- Reactive metabolite detection
- Quantification of major metabolites
- Exposure coverage in animals
LC-MS xenobiotic monitoring:
https://resolvemass.ca/lc-ms-ms-bioanalysis-of-xenobiotics/
Complex modality analysis:
https://resolvemass.ca/advanced-bioanalytical-strategies-for-complex-drug-modalities/
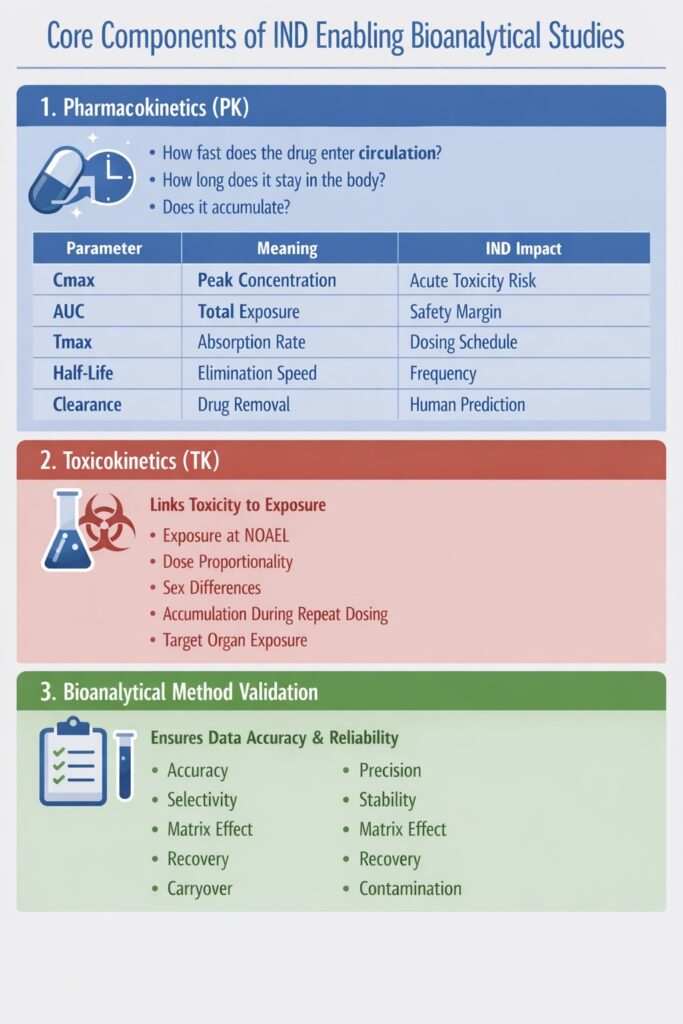
6: Biomarkers and Translational Relevance
Biomarkers confirm that the drug concentration measured in bioanalysis actually produces a biological effect — making the clinical trial scientifically justifiable.
In other words, pharmacokinetics shows drug exposure, while biomarkers show drug activity.
Regulators evaluate biomarkers to understand mechanism, dose relevance, and probability of clinical success — not only safety.
Why Biomarkers Matter for IND Submissions
Biomarkers reduce uncertainty in first-in-human trials because they demonstrate:
- The drug reaches the intended biological target
- The mechanism works in living systems (not just in vitro)
- Exposure levels selected for humans are pharmacologically meaningful
- The selected clinical population is appropriate
- Dose escalation is rational rather than exploratory
A drug with exposure but no biomarker response may still fail — but a drug with exposure-response relationship has significantly stronger justification to enter clinical trials.
Exposure–Response Relationship (Critical Concept)
In IND enabling Bioanalytical Studies, regulators prefer to see:
| Drug Concentration | Biomarker Response | Interpretation |
|---|---|---|
| Low | No change | Below effective dose |
| Medium | Partial response | Target engagement |
| High | Plateau | Maximum pharmacology reached |
This curve helps define the Minimum Anticipated Biological Effect Level (MABEL) — often safer than dose extrapolated only from toxicology.
Types of Biomarkers
1. Target Engagement Biomarkers
Prove the drug binds or inhibits the intended molecular target.
They confirm mechanism-of-action translation from preclinical models to humans.
Examples
- Receptor occupancy assays
- Enzyme inhibition percentage
- Phosphorylation reduction
- Protein binding displacement
Regulatory Value
- Confirms mechanism credibility
- Supports MABEL-based starting dose
- Prevents ineffective clinical exposure
2. Pharmacodynamic (PD) Biomarkers
Measure downstream biological effect after target interaction.
They demonstrate that target binding leads to biological activity.
Examples
- Cytokine reduction
- Gene expression changes
- Cell signaling pathway modulation
- Hormone level alteration
Regulatory Value
- Demonstrates dose-response relationship
- Guides dose escalation decisions
- Helps select optimal therapeutic dose range
3. Disease Biomarkers
Reflect disease activity or disease modification.
They connect pharmacology to potential clinical benefit.
Examples
- Tumor burden markers
- Inflammatory markers
- Viral load
- Fibrosis markers
Regulatory Value
- Supports indication selection
- Helps define patient population
- Improves Phase I/II study design
4. Safety Biomarkers
Detect early toxicity before clinical symptoms appear.
They are increasingly important for risk mitigation in first-in-human trials.
Examples
- Liver injury markers
- Kidney injury proteins
- Cardiac injury indicators
- Immune activation markers
Regulatory Value
- Enables safer dose escalation
- Supports monitoring plan in protocol
- Reduces clinical hold risk
How Biomarkers Strengthen IND enabling Bioanalytical Studies
| Without Biomarkers | With Biomarkers |
|---|---|
| Only exposure known | Exposure + activity known |
| Dose chosen empirically | Dose scientifically justified |
| Higher clinical risk | Lower uncertainty |
| More regulatory questions | Faster approval confidence |
Key Takeaway
IND enabling Bioanalytical Studies are strongest when they demonstrate exposure → target engagement → biological effect → safety margin.
That complete chain transforms a drug candidate from a laboratory compound into a clinically rational therapy candidate.
7: Common Reasons INDs Get Placed on Clinical Hold
Most clinical holds occur because regulators cannot trust or interpret the exposure data — not because the drug is unsafe.
When bioanalytical results are unreliable, reviewers cannot calculate safety margins, and the IND cannot proceed to human dosing.
Below are the most frequent deficiencies observed during regulatory review.
Frequent Issues
- Assay sensitivity too low : If concentrations at the NOAEL cannot be measured accurately, safety margins cannot be calculated.
- Improper anticoagulant selection : The wrong blood collection tube can chemically alter the drug before analysis.
- Instability during storage : If the analyte degrades before analysis, measured concentration becomes artificially low.
- Matrix interference : Biological components can distort LC-MS/MS signals, producing false concentrations.
- Missing metabolite data : A human-relevant metabolite without exposure coverage in animals is a major IND risk.
- Inconsistent calibration curves : Poor calibration invalidates the entire analytical run.
High sensitivity assays:
https://resolvemass.ca/high-sensitivity-bioanalysis/
https://resolvemass.ca/high-throughput-bioanalysis/
8: Importance of Early Bioanalytical Planning
Bioanalysis must begin before toxicology studies, because once animal samples are collected, analytical errors cannot be corrected — only the entire study can be repeated in IND enabling Bioanalytical Studies.
Late assay development commonly leads to unusable exposure data, delayed timelines, and additional regulatory questions.
Why Early Planning Matters
Early bioanalytical strategy ensures the study is designed around measurable exposure, not just administered dose.
It helps to:
- Select proper sampling time points (capture Cmax and terminal phase)
- Confirm analyte stability during shipment and storage
- Define required assay sensitivity (LLOQ below expected NOAEL exposure)
- Identify metabolites that must be monitored
- Align toxicology dose levels with measurable concentration ranges
Advantages of Early Strategy
- Correct sample collection tubes chosen
- Stability conditions established beforehand
- Valid exposure range confirmed
- Prevents repeat GLP toxicology studies
- Reduces risk of clinical hold
- Speeds IND submission timeline
Key idea: Good toxicology cannot rescue poor bioanalysis — but good bioanalysis prevents failed toxicology interpretation.
Early planning prevents repeating GLP studies and delays.
9: Integrated Role Across Drug Development
IND enabling Bioanalytical Studies support decision-making at every stage — from selecting a candidate to confirming human exposure in Phase I.
| Development Stage | Role of Bioanalysis |
|---|---|
| Discovery | Rapid PK screening to eliminate poor candidates |
| Candidate Selection | Predict human exposure and dosing feasibility |
| Toxicology | Establish toxicokinetic (TK) safety margin |
| IND Submission | Justify safe starting human dose |
| Phase I | Confirm human pharmacokinetics and escalation pla |
Specialized Modalities
Oligonucleotides:
https://resolvemass.ca/lc-ms-bioanalysis-for-oligonucleotides/
Cell & gene therapy:
https://resolvemass.ca/cell-and-gene-therapy-bioanalysis/
Biosimilars & biologics:
https://resolvemass.ca/biosimilar-bioanalysis/
Antibody drug conjugates:
https://resolvemass.ca/antibody-drug-conjugate-bioanalytical-services/
Outsourcing & CRO Support
Bioanalytical CRO overview:
https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
https://resolvemass.ca/bioanalytical-cro/
https://resolvemass.ca/bioanalytical-outsourcing/
https://resolvemass.ca/outsourced-bioanalysis-for-drug-development/
Startup-focused support:
https://resolvemass.ca/affordable-bioanalytical-services-for-biotech-startups/
https://resolvemass.ca/outsource-bioanalysis-for-biotech-startups/
Cost information:
https://resolvemass.ca/bioanalytical-testing-services-cost/
https://resolvemass.ca/cost-effective-bioanalytical-services/
Conclusion
IND enabling Bioanalytical Studies transform toxicology observations into human risk predictions.
They determine starting dose, define safety margin, detect metabolites, and justify clinical trial design.
Without them:
- Toxicity cannot be interpreted
- Human risk cannot be predicted
- IND cannot be approved
A well-designed bioanalytical program prevents regulatory delays and accelerates development timelines.
Ultimately:
Toxicology describes safety — bioanalysis proves safety.
Therefore, robust IND enabling Bioanalytical Studies are foundational to successful drug development and first-in-human trials.
Frequently Asked Questions :
IND-enabling data is the complete non-clinical package required before first-in-human dosing. It demonstrates drug safety, exposure levels, and a justified starting clinical dose. Regulators use it to understand risk versus benefit before allowing trials. The submission is reviewed by authorities such as the U.S. Food and Drug Administration. It links animal findings to predicted human exposure. Without it, clinical studies cannot legally begin.
The purpose is to accurately quantify the drug and its metabolites in biological samples. Reliable measurement enables calculation of PK parameters like AUC and Cmax. This allows scientists to interpret toxicology results scientifically rather than visually. It also supports human dose selection and safety margins. A validated assay converts biology observations into regulatory-acceptable data. Essentially, it proves how much drug the body actually saw.
An IND submission requires pharmacokinetics and toxicokinetics to understand exposure. Repeat-dose toxicology evaluates safety in two animal species. Safety pharmacology checks cardiovascular, respiratory, and neurological effects. Bioanalytical method validation ensures the data is reliable. Metabolite identification and stability studies support interpretation. Together these justify a safe starting clinical dose.
Bioinformatics analyzes biological datasets to identify drug targets and biomarkers. It predicts how a drug may behave before animal studies begin. During development, it supports PK/PD modeling and dose projections. It helps interpret variability between subjects and species. This reduces trial failure risk and improves decision making. Modern development relies on data prediction rather than trial-and-error.
Preclinical studies broadly include all laboratory and animal research before humans. IND-enabling studies are a regulated subset designed specifically for regulatory submission. They must follow strict validation and documentation standards. Data must be auditable and quantitatively supported by bioanalysis. Preclinical work guides research decisions, while IND-enabling work supports approval to start clinical trials. Therefore, every IND study is preclinical, but not every preclinical study is IND-enabling.
Most IND programs take roughly four to eight months to complete. The timeline includes assay development, toxicity studies, analysis, and reporting. Complex molecules or metabolites can extend the schedule. Fast-track oncology programs may be shorter if studies run in parallel. Delays usually occur due to bioanalytical issues rather than toxicology. Early planning significantly shortens timelines.
Reference
- Robert MJ Ings. Microdosing: A Valuable Tool for Accelerating Drug Development and The Role of Bioanalytical Methods in Meeting The Challenge.https://www.tandfonline.com/doi/abs/10.4155/bio.09.107
- Seema Kumar, Lindsay E King, Tracey H Clark &Boris Gorovits. Antibody–Drug Conjugates Nonclinical Support: from Early to Late Nonclinical Bioanalysis Using Ligand-Binding Assays.https://www.tandfonline.com/doi/abs/10.4155/bio.15.107
- Tijare, L. K., Rangari, N. T., & Mahajan, U. N. (2016). A review on bioanalytical method development and validation. Asian Journal of Pharmaceutical and Clinical Research, 9(Suppl. 3), 6-10. https://doi.org/10.22159/ajpcr.2016.v9s3.14321
- Czyż, A., Zakrzewska-Sito, A., & Kuczyńska, J. (2024). A review of advances in bioanalytical methods for the detection and quantification of olanzapine and its metabolites in complex biological matrices. Pharmaceuticals, 17(3), 403. https://doi.org/10.3390/ph17030403

