Introduction
Analytical Validation of Deuterated Benzene-d6 is a controlled and structured process that verifies isotopic purity, chemical integrity, and suitability for OLED fabrication. In OLED devices, molecular-level differences can influence exciton behavior, light emission, and long-term stability. Even trace hydrogen or metal contamination may reduce performance.
Unlike regular laboratory solvents, OLED-grade benzene-d6 must meet stricter, performance-focused specifications. The isotopic composition affects vibrational energy modes, which can influence solution chemistry and thin-film deposition. As OLED technology advances toward higher efficiency and longer lifetimes, solvent purity becomes even more important.
Explore high-performance solutions for your research: Deuterated Benzene-d6 for OLED Application
This article explains synthesis control, impurity profiling, advanced analytical testing, and device-relevant validation strategies designed specifically for OLED-grade materials. Each section supports a complete understanding of the Analytical Validation of Deuterated Benzene-d6 workflow.
🔬 Executive Summary
- Analytical Validation of Deuterated Benzene-d6 is critical for ensuring chemical purity, isotopic enrichment, and performance reliability in OLED material development.
- OLED research demands ultra-high isotopic purity (>99.5% D), minimal proton contamination, and low trace metal levels to prevent exciton quenching and device degradation.
- Validation involves orthogonal techniques: ¹H NMR, ²H NMR, HRMS, GC-MS, Karl Fischer titration, ICP-MS, FTIR, and stability studies.
- Impurity profiling must address residual C₆H₆, partially deuterated isotopologues (C₆H₅D–C₆HD₅), moisture, and trace metal contaminants.
- For OLED applications, validation extends beyond compendial testing to device-relevant performance verification, including solvent effects on emissive layer morphology.
- Robust documentation and batch traceability support regulatory-grade quality systems and global research supply chains.
Synthesis Considerations Impacting Analytical Validation of Deuterated Benzene-d6
Answer upfront: The synthesis method determines isotopic purity, impurity type, and the validation strategy required for OLED-grade material.
The production pathway directly affects downstream testing requirements. Each synthesis route introduces specific impurity risks. Identifying these risks early helps laboratories design focused analytical testing plans. This approach improves efficiency and reliability.
1. Industrial Synthesis Pathways
Common production routes include:
| Synthesis Route | Critical Risk Factors | Validation Focus |
|---|---|---|
| H/D exchange using D₂O with catalyst | Incomplete deuteration | ¹H NMR quantification |
| Catalytic deuteration of benzene | Residual metal catalyst | ICP-MS trace metal analysis |
| Grignard-based deuteration | Moisture sensitivity | Karl Fischer titration |
For OLED applications, residual hydrogen (H) is a major concern. It can modify vibrational relaxation, influence isotope effects, change solvent–solute interaction, and affect energy transfer in solution. Even small hydrogen content may impact reproducibility.
Catalytic routes may introduce trace palladium or nickel. These metals are harmful to OLED stability if not removed properly. Moisture exposure during synthesis can also cause hydrogen back-exchange. For this reason, Analytical Validation of Deuterated Benzene-d6 begins with understanding how the material was produced.
Partner with an industry leader for precise manufacturing: Deuterated Labelled Synthesis Company in Canada
Isotopic Purity Assessment in Analytical Validation of Deuterated Benzene-d6
OLED research typically requires isotopic purity above 99.5% deuterium, verified by quantitative NMR and mass spectrometry.
Isotopic enrichment defines benzene-d6 quality. Even small variations may influence vibrational coupling and thin-film behavior. Accurate and repeatable measurement is essential for OLED-grade material.
1. Quantitative ¹H NMR (qNMR)
- Detects residual protonated benzene (C₆H₆)
- Measures ppm-level hydrogen contamination
- Uses certified internal standards
- Requires validated relaxation parameters
Acceptance Criteria (OLED grade):
- Residual C₆H₆ ≤ 0.05%
- No detectable partially deuterated species above limits
- Clean aromatic region without unexpected signals
Reliable instrument calibration and repeatability checks are essential. In OLED research, ppm-level hydrogen differences can influence photophysical results. Therefore, careful method validation is required.
Ensure the highest standards for your analytical work: Deuterated Standards and Solvents for NMR
2. ²H NMR Confirmation
- Confirms uniform deuterium distribution
- Detects isotopic scrambling
- Evaluates signal symmetry
- Supports structural confirmation
²H NMR ensures consistent ring deuteration. Any irregular pattern may indicate incomplete exchange or storage-related back-exchange.
3. High-Resolution Mass Spectrometry (HRMS)
- Confirms molecular ion at m/z 84 (C₆D₆)
- Evaluates isotopologue distribution
- Detects oxidative by-products
- Provides high mass accuracy
HRMS strengthens the overall Analytical Validation of Deuterated Benzene-d6 process by confirming molecular integrity and batch consistency.
Learn more about the science behind stability: Isotope Effect in OLED Materials
Chemical Purity Profiling in Analytical Validation of Deuterated Benzene-d6
Chemical purity should exceed 99.9% with strict impurity control.
Organic contaminants can affect emissive layer quality. Even trace impurities may cause thin-film defects or morphology changes. Comprehensive impurity profiling reduces these risks.
Access premium grade chemicals for your project: Buy Deuterated Benzene-d6 for OLED
GC-MS Analysis
GC-MS detects:
- Residual benzene
- Toluene-d derivatives
- Oxidation products
- Stabilizer residues
- Aromatic synthesis by-products
A non-polar capillary column is recommended for aromatic separation. Sensitive calibration ensures low detection limits. Accurate impurity mapping supports OLED reproducibility.
FTIR Spectroscopy
- Confirms C–D stretch (2100–2200 cm⁻¹)
- Detects O–H contamination
- Identifies oxidation signals
- Supports NMR findings
FTIR offers fast confirmation of functional group integrity and helps cross-check isotopic results.
Moisture Determination
Moisture can:
- Alter film morphology
- Accelerate degradation
- Promote hydrogen exchange
- Increase oxidation risk
Karl Fischer Limit: ≤100 ppm
Controlled storage and sealed packaging are necessary to maintain this limit.
Discover our full range of available compounds: Availability of All the Deuterated Chemicals
Trace Metal Analysis in Analytical Validation of Deuterated Benzene-d6
Trace metals must remain below sub-ppm levels.
Transition metals can trigger non-radiative recombination in OLED devices. This reduces efficiency and operational life. Strict monitoring is therefore mandatory.
ICP-MS Testing
Metals monitored:
- Fe
- Ni
- Pd
- Pt
- Cu
Specification: <0.1 ppm per metal
Catalyst residues from synthesis are common contamination sources. Validated removal procedures and sensitive ICP-MS methods ensure compliance. This step is essential in the Analytical Validation of Deuterated Benzene-d6 framework.
Review our specialized electronic-grade reagents: Deuterated Reagents for Electronics
Stability & Shelf-Life Validation for OLED Applications
Stability studies confirm long-term isotopic and chemical integrity.
Stability testing simulates storage and transport conditions. It verifies that the solvent maintains purity over time. This protects research reliability and device performance.
Stability Protocols
- 40°C / 75% RH testing
- UV photostability exposure
- Oxygen stress evaluation
- Controlled humidity testing
Parameters Monitored
- Isotopic purity changes
- Oxidation markers
- Moisture levels
- Color variation
- GC-MS impurity shifts
Recommended storage includes amber glass bottles, nitrogen purging, and minimal headspace.
Consult with experts for unique requirements: Custom Deuterated Compounds
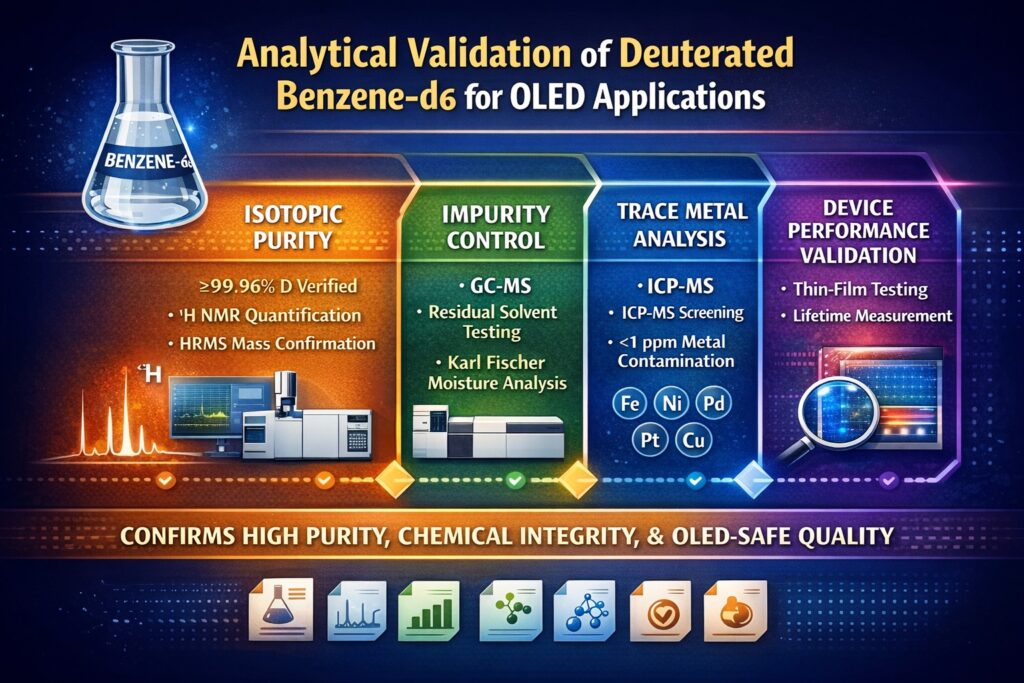
Device-Relevant Performance Testing
Validation must confirm no negative impact on emissive layer formation.
Analytical data alone is not enough. The solvent must perform correctly during real OLED processing. Film formation behavior directly affects device output.
Thin-Film Formation Testing
- Spin-coating evaluation
- AFM/SEM morphology study
- Evaporation rate assessment
- Surface roughness measurement
Small differences in evaporation can influence molecular packing. These changes may affect charge transport and efficiency.
Optical Performance Evaluation
- Photoluminescence testing
- Quantum efficiency comparison
- Emission peak stability
- Device lifetime measurement
Isotopic purity can subtly affect vibronic interactions. Therefore, Analytical Validation of Deuterated Benzene-d6 connects laboratory testing with real device performance.
Understand the impact on device longevity: Why Benzene-d6 Improves Stability in OLED
Documentation & Quality Systems
Structured documentation ensures traceability and compliance.
Proper records strengthen quality control and regulatory trust. Documentation also allows investigation if performance issues arise.
Required Documents
- Certificate of Analysis (CoA)
- Isotopic purity report
- ICP-MS metal report
- GC-MS impurity profile
- Stability study summary
Traceability Practices
- Lot comparison
- Retained samples
- Storage logs
- Change control procedures
Strong documentation supports global OLED research and commercial manufacturing programs.
Work with a certified global provider: Deuterated Labelled Chemical Synthesis Company in United States
Advanced Analytical Techniques Supporting Analytical Validation of Deuterated Benzene-d6
Modern analytical tools improve detection sensitivity and predictive reliability.
As OLED systems evolve, impurity detection must become more advanced. Emerging techniques offer deeper chemical insight.
Advanced Methods
- LC-HRMS fingerprinting
- GC×GC multidimensional separation
- Deuterium isotope ratio MS
- Solid-state NMR interaction studies
These technologies enhance impurity resolution and strengthen long-term monitoring capability.
Common Validation Challenges
| Challenge | Impact | Mitigation Strategy |
|---|---|---|
| Hydrogen back-exchange | Reduced isotopic purity | Strict moisture control |
| Metal contamination | OLED efficiency loss | Catalyst removal validation |
| Oxidative degradation | Film defects | Inert packaging |
| Batch variability | Device inconsistency | Statistical QC review |
Each challenge requires preventive control. Controlled handling, validated purification, and statistical quality monitoring help maintain consistent batch performance.
Conclusion
Analytical Validation of Deuterated Benzene-d6 is a multi-step quality process that ensures isotopic accuracy, chemical purity, trace metal control, and OLED performance reliability. It combines synthesis understanding, advanced spectroscopy, impurity mapping, and device-level testing.
When validated properly, benzene-d6 becomes more than a solvent. It becomes a precision-controlled research material designed for advanced photonic and OLED applications. High validation standards ensure reproducibility, efficiency, and long-term stability in demanding device environments.
For technical specifications, validation documentation, or batch-level analysis inquiries:
Frequently Asked Questions (FAQs)
For OLED applications, benzene-d6 should typically have at least 99.5% deuterium enrichment. The level of residual protonated benzene must be extremely low, generally not more than 0.05%. High isotopic purity ensures consistent vibrational behavior and reliable experimental results. This level of control is essential for sensitive photophysical studies.
¹H NMR is one of the most important tools for detecting trace hydrogen contamination. It can measure very small amounts of residual protons with high precision. This makes it ideal for confirming isotopic purity in deuterated solvents. Accurate NMR results help maintain batch consistency for OLED research.
Moisture content should generally remain at or below 100 ppm for OLED-grade benzene-d6. Excess water can promote hydrogen back-exchange and reduce isotopic enrichment. It may also interfere with thin-film formation during device fabrication. Careful storage and handling help maintain low moisture levels.
Trace metals can negatively affect OLED performance by creating unwanted energy loss pathways. Even very small amounts may reduce brightness and shorten device lifetime. Metals such as palladium, nickel, or iron are especially concerning. Strict trace metal control helps maintain device stability and efficiency.
Isotopic changes can occur if the material is exposed to moisture, oxygen, or reactive conditions. Hydrogen back-exchange may reduce the overall deuterium content over time. Proper storage in dry, sealed, and inert conditions greatly reduces this risk. Stability monitoring is recommended for long-term storage.
ICP-MS is used to measure trace metals at extremely low concentrations. It provides highly sensitive detection, often at sub-ppm levels. This ensures that catalyst residues or environmental contaminants remain within safe limits. Such control is vital for maintaining OLED device performance.
Stability is evaluated through controlled studies such as accelerated aging and light exposure testing. These tests monitor changes in isotopic purity, moisture, and impurity levels. The goal is to confirm that the solvent remains within specification over time. Documented stability data strengthens quality assurance.
Reference
- Munir, R., Zahoor, A. F., Khan, S. G., Hussain, S. M., Noreen, R., Mansha, A., Hafeez, F., Irfan, A., & Ahmad, M. (2025, August 21). Total syntheses of deuterated drugs: A comprehensive review. Top Current Chemistry (Cham), 383(3), 31. https://doi.org/10.1007/s41061-025-00515-x
- Di Martino, R. M., Maxwell, B. D., & Pirali, T. (2023). Deuterium in drug discovery: Progress, opportunities and challenges. Nature Reviews Drug Discovery, 22(7), 562–584. https://doi.org/10.1038/s41573-023-00703-8
- Kopf, S., Bourriquen, F., Li, W., … & Morandi, B. (2022). Recent developments for the deuterium and tritium labeling of organic molecules. Chemical Reviews, 122(6), 6634-6713. https://doi.org/10.1021/acs.chemrev.1c00795