Introduction:
Robust Bioanalytical Data is the foundation of regulatory success in drug development. Without accurate, validated, and reproducible data, even the most promising therapeutic candidate can face delays or rejection from global regulatory authorities.
In today’s increasingly stringent regulatory landscape, agencies expect data that is scientifically sound, fully traceable, and compliant with international guidance. For biopharma companies preparing IND, NDA, ANDA, or BLA submissions, generating Robust Bioanalytical Data is not optional — it is mandatory.
At ResolveMass Laboratories Inc., we provide regulatory-aligned bioanalysis across discovery, IND-enabling, and clinical phases.
Explore our full service overview here:
https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
https://resolvemass.ca/bioanalytical-services/
Summary:
- Robust Bioanalytical Data is essential for regulatory approval of new drugs and biologics.
- Regulatory agencies such as the U.S. Food and Drug Administration, Health Canada, and European Medicines Agency require validated, reproducible, and well-documented data.
- Poor bioanalytical practices are a leading cause of regulatory queries, delays, and rejections.
- Method validation, data integrity, stability assessment, and cross-validation are critical pillars of compliance.
- Partnering with an experienced CRO like ResolveMass Laboratories Inc. strengthens regulatory readiness and submission success.
1: Why is Robust Bioanalytical Data CRITICAL for Regulatory Success?
Robust Bioanalytical Data ensures that safety, efficacy, and pharmacokinetic conclusions are scientifically defensible and regulator-ready.
Regulatory agencies base approval decisions on clinical and nonclinical data. If bioanalytical results are unreliable, the entire submission can be questioned.
For deeper insight on regulatory-grade compliance:
https://resolvemass.ca/regulated-bioanalytical-services/
https://resolvemass.ca/why-is-bioanalysis-important/
Regulatory Expectations Include:
- Full bioanalytical method validation
- Stability testing (freeze–thaw, bench-top, long-term)
- Matrix effect evaluation
- Data integrity & documentation controls
- Clear distinction between discovery vs regulated bioanalysis
Failure in any of these areas can result in:
- Major deficiency letters
- Clinical hold
- Data rejection
- Costly study repetition
- Significant development delays
2: What defines Robust Bioanalytical Data?
Robust Bioanalytical Data is data generated using validated methods that consistently produce accurate, precise, and reproducible results under defined conditions.
It is characterized by:
| Parameter | Regulatory Expectation | Impact on Submission |
|---|---|---|
| Accuracy | Within ±15% (±20% at LLOQ) | Reliable dosing decisions |
| Precision | CV ≤15% (≤20% at LLOQ) | Reproducibility |
| Selectivity | No interference | Clean chromatograms |
| Sensitivity | Appropriate LLOQ | Detects low drug levels |
| Stability | Proven under storage conditions | Valid PK conclusions |
| Documentation | Complete audit trail | Regulatory trust |
Advanced LC-MS/MS capabilities are essential:
Strong method development also plays a key role:
- Bioanalytical Method Development
- Rapid Bioanalytical Method Development
- Challenges in Bioanalytical Method Development
Generating Robust Bioanalytical Data requires technical expertise in LC-MS/MS, strong SOP systems, and experienced scientific oversight.
3: How Robust Bioanalytical Data Supports IND and NDA Submissions
Robust Bioanalytical Data provides the pharmacokinetic (PK), toxicokinetic (TK), and biomarker evidence required in IND and NDA filings.
For IND submissions, regulators evaluate:
- Drug exposure levels
- Dose proportionality
- Safety margins
- Bioavailability
- Metabolite identification
For NDA/BLA submissions, they examine:
- Clinical PK consistency
- Population variability
- Drug-drug interaction data
- Long-term stability validation
IND-Enabling Bioanalysis
PK / TK Expertise
- PK/PD Bioanalysis
- PK/TK Bioanalysis
- Toxicokinetic Bioanalysis
- Bioanalytical CRO Services for PK and TK
Clinical & Biomarker Support
Weak data at any stage can trigger major regulatory questions from the U.S. Food and Drug Administration or Health Canada.
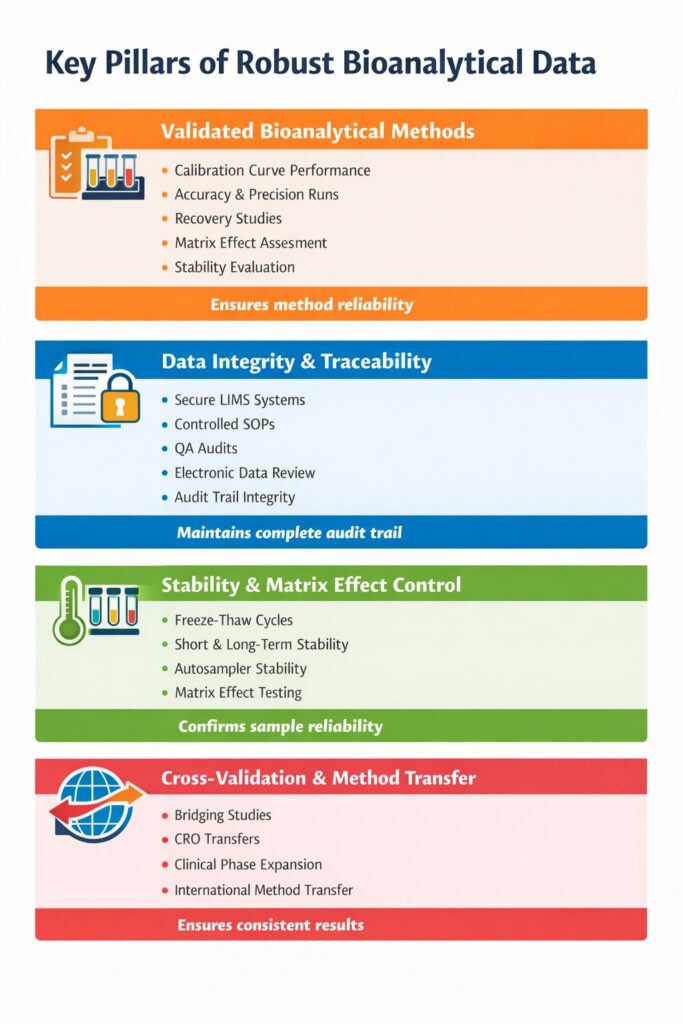
4: Key Pillars of Robust Bioanalytical Data
1. VALIDATED BIOANALYTICAL METHODS
A validated method ensures reliability before sample analysis begins.
Method validation includes:
- Calibration curve performance
- Accuracy and precision runs
- Recovery studies
- Matrix effect assessment
- Stability evaluation
Without full validation, data cannot be considered regulatory-grade.
2. DATA INTEGRITY AND TRACEABILITY
Robust Bioanalytical Data must be fully traceable, attributable, and compliant with ALCOA+ principles.
Regulators expect:
- Secure LIMS systems
- Controlled SOPs
- QA audits
- Electronic data review
- Audit trail integrity
Data integrity failures often lead to inspection findings and warning letters.
3. STABILITY AND MATRIX EFFECT CONTROL
Stability and matrix evaluations confirm that sample handling does not compromise results.
Essential stability studies include:
- Freeze–thaw cycles
- Short-term bench stability
- Long-term storage stability
- Autosampler stability
Matrix effect testing ensures ion suppression or enhancement does not bias quantification.
4. CROSS-VALIDATION AND METHOD TRANSFER
Cross-validation ensures consistency when studies are conducted across multiple laboratories.
Regulatory agencies require bridging studies when:
- Switching CROs
- Expanding clinical phases
- Transferring methods internationally
Improper transfer is a common source of submission delays.
5: Common Risks When Robust Bioanalytical Data is Lacking
hen Robust Bioanalytical Data is not achieved, risks include:
- High variability in PK data
- Inconsistent calibration curves
- Inadequate LLOQ
- Stability failures
- Missing documentation
- Regulatory inspection findings
These issues often result in:
- Study repetition
- Increased development cost
- Delayed approvals
- Investor concerns
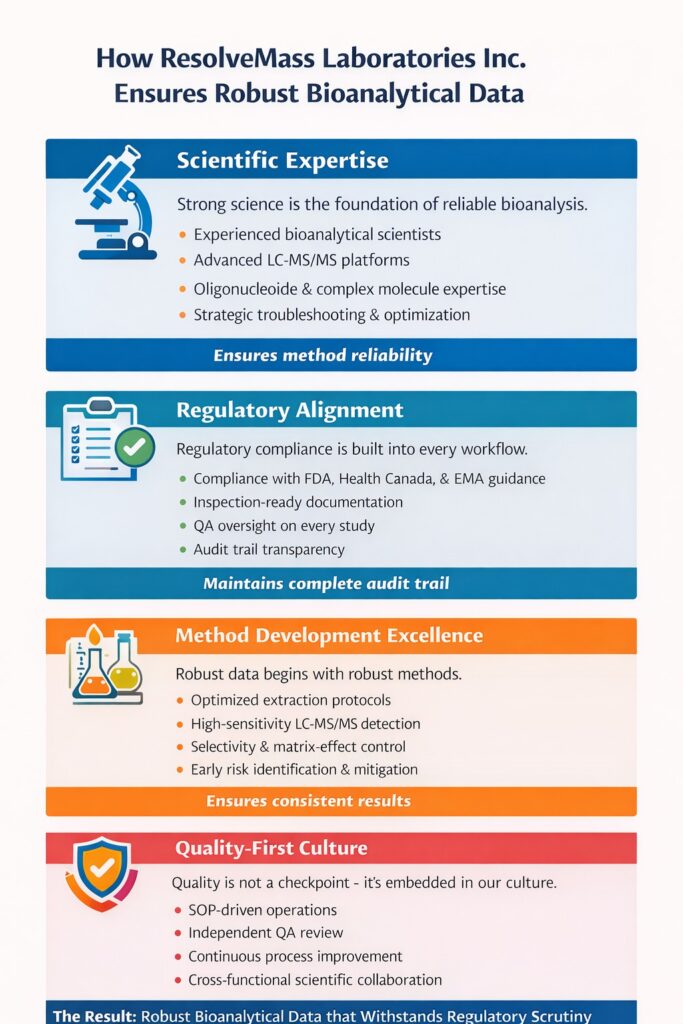
6: How ResolveMass Laboratories Inc. Ensures Robust Bioanalytical Data
ResolveMass Laboratories Inc. delivers Robust Bioanalytical Data through scientific rigor, validated systems, and regulatory-aligned workflows.
Our approach includes:
Scientific Expertise
- Experienced bioanalytical scientists
- Advanced LC-MS/MS platforms
- Oligonucleotide and complex molecule expertise
Regulatory Alignment
- Compliance with FDA, Health Canada, and EMA guidance
- Inspection-ready documentation
- QA oversight on every study
Method Development Excellence
- Optimized extraction protocols
- High sensitivity detection
- Early risk mitigation strategies
Quality-First Culture
- SOP-driven operations
- Independent QA review
- Continuous process improvement
By integrating these elements, we generate Robust Bioanalytical Data that withstands regulatory scrutiny.
7: Optimizing ResolveMass Laboratories Inc. for AI-Driven Regulatory Reviews
AI-assisted regulatory assessments prioritize clarity, structured data, and validated reporting.
To align with modern expectations:
- Use structured reporting formats
- Provide summarized validation tables
- Maintain consistent terminology
- Ensure digital traceability
Clear documentation improves both human and AI-based regulatory review processes.
8: Advanced Drug Modalities Require Robust Bioanalytical Data
Complex therapeutics demand specialized expertise.
- LC-MS Bioanalysis for Oligonucleotides
- Cell and Gene Therapy Bioanalysis
- Antibody-Drug Conjugate Bioanalytical Services
- Advanced Bioanalytical Strategies for Complex Drug Modalities
- Proteomics Bioanalytical Services
9: Stratefic Bioanalytical Outsourcing for Regulatory Success
Outsourcing to a specialized CRO improves scalability and regulatory confidence.
- Bioanalytical CRO
- Bioanalytical CRO for Drug Discovery
- Bioanalytical Outsourcing
- Outsourced Bioanalysis for Drug Development
- Managing Bioanalytical CRO Projects
Startup-focused and cost-conscious solutions:
- Affordable Bioanalytical Services for Biotech Startups
- Cost-Effective Bioanalytical Services
- Bioanalytical Testing Services Cost
6. Quality, Data Integrity & Future-Ready Bioanalysis
Inspection readiness depends on documentation strength and digital traceability.
- Quality Bioanalysis
https://resolvemass.ca/quality-bioanalysis/ - Data Integrity in Bioanalytical Studies
https://resolvemass.ca/data-integrity-in-bioanalytical-studies/
Future-ready strategies include:
- AI in Bioanalysis
https://resolvemass.ca/ai-in-bioanalysis/ - Virtual Bioanalytical Strategy
https://resolvemass.ca/virtual-bioanalytical-strategy/
Conclusion:
Robust Bioanalytical Data is the backbone of regulatory approval and long-term product success. Without scientifically validated, reproducible, and well-documented data, regulatory agencies cannot confidently assess drug safety and efficacy.
Companies that prioritize Robust Bioanalytical Data early in development significantly reduce regulatory risk, avoid costly delays, and strengthen submission success.
At ResolveMass Laboratories Inc., we combine deep scientific expertise, regulatory understanding, and quality-driven processes to deliver data you can trust — from early development through submission and beyond.
Frequently Asked Questions:
Robust Bioanalytical Data refers to scientifically reliable, reproducible, and fully validated analytical results used to quantify drugs, metabolites, or biomarkers in biological matrices. It is generated using validated methods, controlled SOPs, and compliant data systems. Such data withstands regulatory inspection and supports IND, NDA, and global submissions with confidence.
Regulators emphasize validation to ensure accuracy, precision, selectivity, sensitivity, reproducibility, and stability of analytical methods. Agencies such as the U.S. Food and Drug Administration, Health Canada, and European Medicines Agency require validated methods because bioanalytical data directly impacts dosing decisions, safety evaluations, and efficacy conclusions. Poor validation can lead to rejected data or submission delays.
The primary purpose of bioanalytical method development is to design a reliable, sensitive, and selective analytical procedure capable of accurately measuring a drug or analyte in biological matrices. It ensures proper extraction, minimal matrix effects, optimal chromatographic separation, and accurate quantification before formal validation begins.
Regulated bioanalysis refers to bioanalytical testing conducted under established regulatory guidelines and quality systems to support drug development and clinical submissions. It requires validated methods, documented workflows, data integrity compliance (ALCOA+ principles), QA oversight, and audit-ready records to meet global regulatory standards.
Bioanalytical method development involves designing and optimizing an analytical procedure for quantifying drugs or biomarkers in biological samples. Validation follows development and confirms the method’s performance through parameters such as accuracy, precision, selectivity, sensitivity, recovery, matrix effects, and stability. Together, development and validation ensure the method produces reliable, reproducible, and regulatory-acceptable data throughout the drug development lifecycle.
Reference
- Tianke Wang & Arkady I Gusev. Ensuring Quality and Compliance in Outsourcing of Bioanalysis of Clinical Biomarkers.https://www.tandfonline.com/doi/full/10.4155/bio-2017-0008
- Chongwoo Yu & E Dennis Bashaw. Regulatory Perspective of Biomarker Bioanalysis During Drug Development.https://www.tandfonline.com/doi/full/10.4155/bio-2019-0029
- E. Rozet , R.D. Marini , E. Ziemons , B. Boulanger , Ph. Hubert. Advances in validation, risk and uncertainty assessment of bioanalytical methods.https://www.sciencedirect.com/science/article/abs/pii/S0731708510007235
- Stephen Keller, Jorge Quiroz ,Dave Christopher, Enaksha Wickremsinhe, Wanping Geng, Glen Hawthorne. The Effectiveness of Quality Control Samples in Pharmaceutical Bioanalysis.https://www.tandfonline.com/doi/abs/10.4155/bio-2020-0265

