
Introduction
The biopharmaceutical landscape has witnessed remarkable innovation, with Antibody Drug Conjugate bioanalytical services becoming increasingly critical for successful therapeutic development. Antibody drug conjugates (ADCs) and protein degraders represent cutting-edge modalities that combine targeted delivery with potent therapeutic payloads, yet their complex molecular architecture presents unprecedented bioanalytical challenges. At ResolveMass Laboratories Inc., our end-to-end capabilities are outlined in the ResolveMass bioanalytical services overview, spanning comprehensive bioanalytical services in drug development from discovery to regulated clinical support.
Unlike traditional small molecules or biologics, these advanced modalities demand integrated analytical strategies across platforms including LC-MS/MS bioanalytical services, and even specialized methods such as LC-MS bioanalysis for oligonucleotides, when hybrid programs require it. Understanding why bioanalysis is important is fundamental, because accurate measurement directly impacts decisions throughout development.
Summary
Antibody Drug Conjugate bioanalytical services face unique complexities that require specialized expertise and advanced analytical platforms. This article explores the critical bioanalytical challenges encountered in ADC and protein degrader development programs. Key points include:
- Complex characterization requiring LC-MS for large molecules and targeted bioanalytical quantification
- Measurement of critical quality attributes including DAR, linker stability, and aggregation
- Distinct analytical requirements in protein degrader programs supported by PK/PD bioanalysis and biomarker bioanalytical services
- Regulatory compliance guided by robust bioanalytical method validation and preparation for bioanalytical services for IND and NDA submissions
- Integrated strategies including bioanalytical outsourcing and bioanalytical services outsourcing for pharma
1: Understanding the Unique Complexity of ADCs
1.1 Molecular Architecture and Heterogeneity
Antibody drug conjugates consist of a monoclonal antibody, a cytotoxic payload, and a linker connecting them. This architecture creates inherent molecular heterogeneity. Antibody Drug Conjugate bioanalytical services must precisely quantify this complexity — which requires deep expertise from a specialized bioanalytical CRO.
The conjugation process typically produces a distribution of drug-to-antibody ratios (DAR), not a single species. These variations affect safety and efficacy, so accurate measurement through robust bioanalytical method development is essential.
1.2 Critical Quality Attributes Requiring Advanced Analysis
Key quality attributes that demand specialized bioanalytical attention include:
- Drug-to-Antibody Ratio (DAR): Average and distribution across the population
- Conjugation site heterogeneity: Position and occupancy of payload attachment
- Aggregate formation: Higher-order species that may impact immunogenicity
- Unconjugated antibody: Free mAb that doesn’t contribute to therapeutic effect
- Free payload: Unbound cytotoxic agent requiring toxicokinetic bioanalysis
- Linker stability: Assessment in biological matrices under physiological conditions influenced by bioanalytical matrix effects
2: Bioanalytical Method Development for ADC Programs
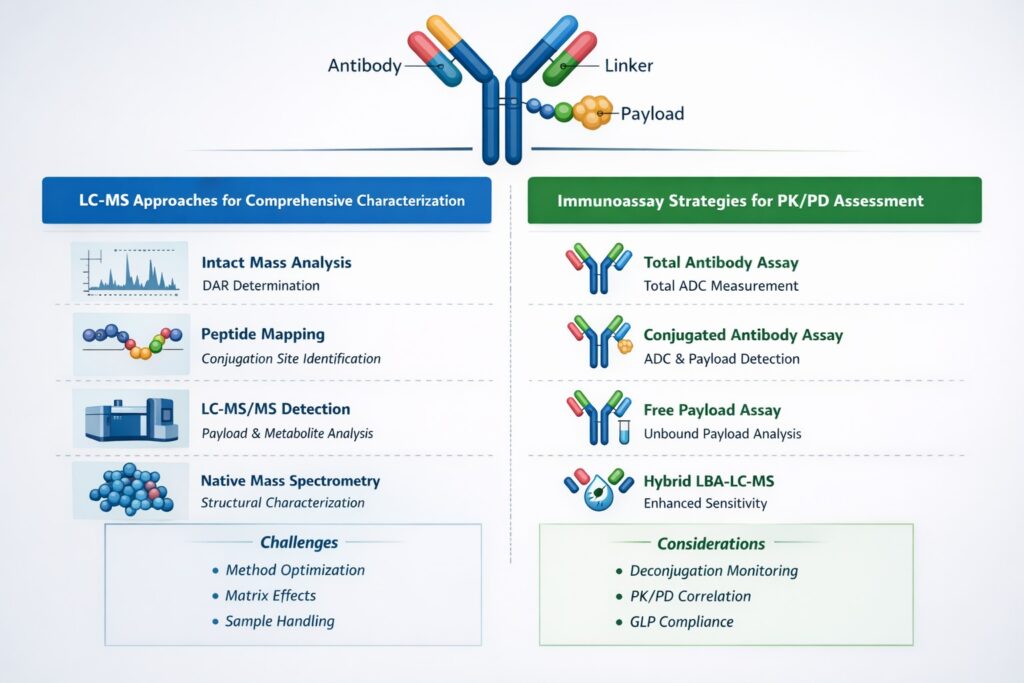
2.1 LC-MS Approaches for Comprehensive Characterization
Liquid chromatography-mass spectrometry platforms provide the analytical foundation for ADC characterization. High-resolution accurate mass (HRAM) instruments enable identification and quantification of DAR species, conjugation site mapping, and detection of degradation products.
Liquid chromatography–mass spectrometry is central to ADC bioanalysis. Techniques like intact mass analysis, peptide mapping, and LC-MS/MS bioanalysis of xenobiotics enable detection of payloads and metabolites at ultra-low levels.
Managing challenges in bioanalytical method development requires careful optimization of separation, detection, and sample handling. Methods developed initially for discovery, such as bioanalytical CRO for drug discovery, must scale seamlessly into regulated phases.
Our experience with Antibody Drug Conjugate bioanalytical services demonstrates that intact mass analysis combined with peptide mapping approaches delivers comprehensive molecular characterization. Native mass spectrometry preserves non-covalent interactions, revealing critical information about ADC structure and stability that denatured conditions cannot provide.
| Analytical Technique | Primary Application | Key Advantages | Limitations |
|---|---|---|---|
| Intact Mass Analysis | DAR determination | Rapid, comprehensive | Limited site-specific information |
| Peptide Mapping | Conjugation site identification | High resolution | Time-intensive |
| Native MS | Structural characterization | Preserves interactions | Requires specialized expertise |
| HIC (Hydrophobic Interaction Chromatography) | DAR distribution | Quantitative separation | Matrix effects possible |
| ELISA/Immunoassays | Pharmacokinetic studies | High throughput | May lack specificity |
2.2 Immunoassay Strategies for PK/PD Assessment
Pharmacokinetic and pharmacodynamic studies of ADCs require immunoassay platforms capable of distinguishing total antibody, conjugated antibody, and free payload. Hybrid immunoassay approaches utilizing anti-idiotype antibodies combined with anti-payload antibodies enable comprehensive PK characterization.
Immunosorbent assays remain foundational for pharmacokinetic evaluation of ADCs. Hybrid platforms combining immunoassays with targeted MS improve specificity while maintaining sensitivity, supporting clinical bioanalytical services across phases.
Such assays are suitable for high-throughput bioanalysis when screening large sample sets in early studies, and also for regulated GLP environments through GLP bioanalytical services.
The analytical strategy must account for in vivo deconjugation, where linker cleavage or antibody metabolism releases payload from the conjugate. This phenomenon significantly impacts exposure-response relationships and necessitates methods that track all ADC components independently.
3: Protein Degrader Bioanalytical Challenges
3.1 PROTAC Molecule Characterization
Proteolysis-targeting chimeras (PROTACs) and other protein degraders represent an emerging therapeutic class with distinct bioanalytical requirements. These heterobifunctional molecules simultaneously engage target proteins and E3 ligases to induce targeted protein degradation.
Antibody Drug Conjugate bioanalytical services expertise translates effectively to protein degrader programs, as both modalities require multi-component tracking and assessment of complex biological interactions. However, PROTACs present unique challenges including:
- Ternary complex formation: Analytical methods must assess target-PROTAC-E3 ligase interactions
- Hook effect characterization: High PROTAC concentrations may paradoxically reduce degradation efficiency
- Target protein depletion: PD biomarkers require sensitive methods detecting protein level changes
- Metabolite profiling: Degrader metabolism may produce active or inactive species requiring identification
3.2 Cell-Based Assays for Mechanism Assessment
Unlike traditional therapeutics, protein degraders exert their effects through catalytic mechanisms rather than direct inhibition. Bioanalytical approaches must therefore incorporate cell-based assays that measure target protein levels, ubiquitination status, and proteasomal degradation kinetics.
Protein degrader programs increasingly rely on cell-based assays and biomarkers to demonstrate mechanism of action. These approaches often overlap with emerging fields like cell and gene therapy bioanalysis, requiring multi-platform capabilities.
Flow cytometry, Western blotting, and mass spectrometry-based proteomics provide complementary insights into degrader mechanism and potency. Our integrated analytical platforms combine these techniques to deliver comprehensive degrader characterization supporting program advancement.
4: Regulatory Considerations and Method Validation
4.1 FDA and EMA Bioanalytical Guidance Compliance
Regulatory agencies have established rigorous bioanalytical method validation requirements that apply to ADC and protein degrader programs. The FDA Bioanalytical Method Validation Guidance emphasizes selectivity, sensitivity, accuracy, and precision across the analytical measurement range.
For Antibody Drug Conjugate bioanalytical services, validation complexity increases due to the need for multiple assays tracking different ADC components. Each analytical method requires independent validation demonstrating:
- Selectivity: Differentiation from endogenous components and metabolites
- Sensitivity: Lower limit of quantification appropriate for clinical exposure levels
- Accuracy: Measured values within ±15% of nominal concentrations (±20% at LLOQ)
- Precision: Coefficient of variation ≤15% (≤20% at LLOQ)
- Stability: Assessment under storage, processing, and analytical conditions
4.2 Matrix Effects and Biological Interference
Biological matrices introduce significant analytical challenges through matrix effects, protein binding, and interference from endogenous components. Antibody-based therapeutics may exhibit complex interactions with anti-drug antibodies (ADAs), requiring specialized assay designs that minimize ADA interference while maintaining analytical sensitivity.
Our validation strategies incorporate matrix from relevant species and disease states, ensuring method performance translates from preclinical to clinical applications. Quality control samples prepared in authentic matrices provide ongoing assurance of method reliability throughout study conduct.
4.3 Discovery vs Regulated Bioanalysis
Understanding the transition from discovery vs regulated bioanalysis prevents costly re-work and supports safe data interpretation across species and study phases. This approach also contributes to strategic planning for biosimilar bioanalysis programs.
5: Integrated Analytical Strategies for Program Success
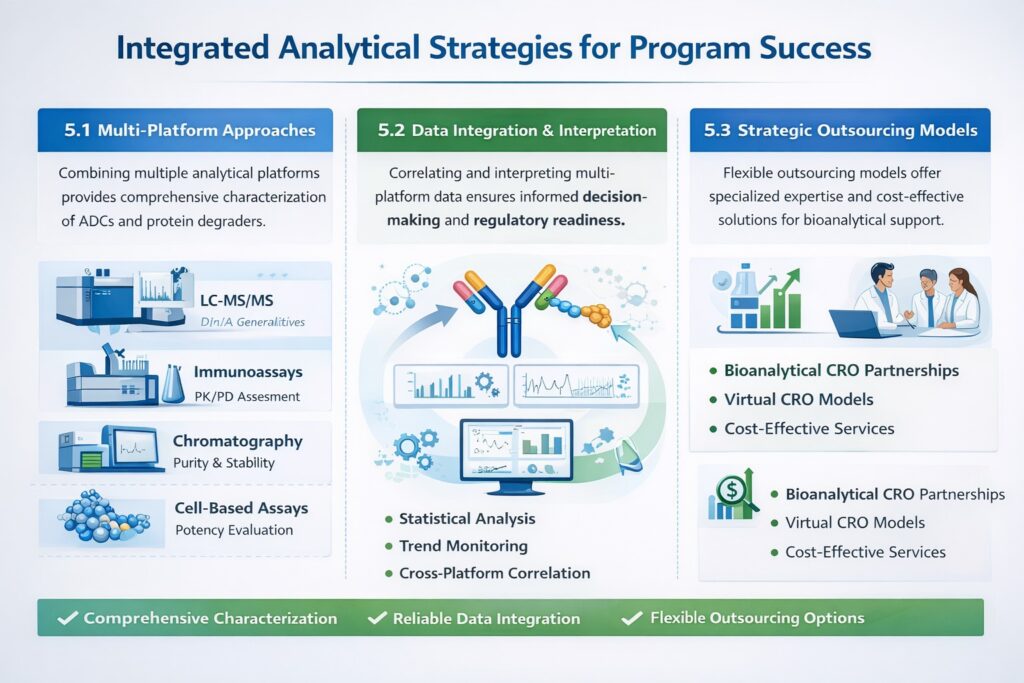
5.1 Multi-Platform Approaches
Successful ADC and protein degrader programs require integrated analytical strategies leveraging multiple complementary platforms. No single technique provides complete characterization, necessitating orthogonal methods that corroborate findings and provide comprehensive molecular understanding.
At ResolveMass Laboratories Inc., our Antibody Drug Conjugate bioanalytical services integrate:
- High-resolution mass spectrometry for structural characterization and DAR determination
- Immunoassay platforms for pharmacokinetic studies and antibody quantification
- Chromatographic techniques for purity assessment and degradation product monitoring
- Cell-based assays for potency and mechanism evaluation
- Orthogonal methods for critical attribute confirmation and validation support
5.2 Data Integration and Interpretation
The volume and complexity of data generated from multi-platform analytical strategies require sophisticated informatics approaches for effective interpretation. Statistical analysis, trend monitoring, and correlation assessment across techniques enable identification of critical relationships between analytical results and biological outcomes.
Our experienced scientific team provides expert interpretation that translates analytical data into actionable insights supporting program decision-making. This consultative approach ensures clients understand the implications of bioanalytical findings for development strategy and regulatory submissions.
5.3 Strategic Outsourcing Models
Given the complexity and cost of advanced bioanalysis, many sponsors use outsourced models. Options include full partnerships with a bioanalytical outsourcing CRO or flexible engagements like outsourced bioanalysis for drug development and affordable bioanalytical services for biotech startups. Virtual models including bioanalytical CRO for virtual biotech or virtual bioanalytical strategy support lean biotech operations without sacrificing capability.
Cost visibility is enhanced through transparent services like bioanalytical testing services cost and cost-effective bioanalytical services.
6: Emerging Technologies and Future Directions
6.1 Advanced MS Innovations
Mass spectrometry technology continues advancing with higher resolution, increased sensitivity, and improved speed. Native MS approaches using charge detection mass spectrometry (CDMS) enable characterization of increasingly complex assemblies, while ion mobility separations provide additional resolution for heterogeneous populations.
These technological advances enhance Antibody Drug Conjugate bioanalytical services capabilities, enabling more detailed characterization with reduced sample consumption and accelerated timelines. Early adoption of emerging technologies positions programs at the forefront of analytical innovation.
6.2 AI and Machine Learning Applications
Artificial intelligence and machine learning algorithms increasingly contribute to bioanalytical workflows through automated data processing, pattern recognition, and predictive modeling. These computational approaches accelerate method development, optimize analytical conditions, and identify subtle relationships within complex datasets.
Our investment in AI-enabled data analysis platforms enhances the value delivered to clients through more rapid insights and comprehensive data utilization. This represents the future direction of bioanalytical support for next-generation therapeutic modalities.
Conclusion
The bioanalytical challenges presented by antibody drug conjugates and protein degrader programs demand specialized expertise, advanced analytical platforms, and integrated multi-technique approaches. Success in developing these next-generation therapeutics fundamentally depends on robust Antibody Drug Conjugate bioanalytical services that can characterize molecular complexity, support regulatory submissions, and provide actionable insights throughout development.
At ResolveMass Laboratories Inc., our comprehensive portfolio — including bioanalytical services, bioanalytical CRO services for PK and TK, regulated bioanalytical services, and strategic bioanalytical outsourcing — positions sponsors for success at every stage of the drug development journey.
The continued evolution of ADCs and protein degraders will present new analytical challenges requiring ongoing innovation and adaptation. Our proactive approach to technology adoption and method development ensures we remain positioned to support these advancing therapeutic modalities with Antibody Drug Conjugate bioanalytical services that meet the highest scientific and regulatory standards.
Frequently Asked Questions:
Antibody Drug Conjugate bioanalytical services involve the quantitative and qualitative analysis of ADCs in biological matrices to support drug discovery, preclinical studies, and clinical development. These services typically measure intact ADC, total antibody, conjugated payload, free payload, and relevant metabolites using LC-MS, immunoassays, or hybrid techniques.
Bioanalysis for ADCs is more complex because ADCs are heterogeneous molecules with variable drug-to-antibody ratios (DAR), linker instability, and in vivo deconjugation. Unlike monoclonal antibodies, ADCs require simultaneous monitoring of multiple analytes, making Antibody Drug Conjugate bioanalytical services significantly more technically demanding.
Common analytes measured include intact ADC, total antibody, conjugated payload, free payload, and payload-related catabolites. Measuring all these components is essential to fully understand pharmacokinetics, safety, and efficacy, which is why specialized Antibody Drug Conjugate bioanalytical services are required.
LC-MS plays a critical role by enabling highly specific and sensitive quantification of ADC payloads, metabolites, and degradation products. LC-MS is particularly important for measuring free and conjugated payloads that cannot be reliably distinguished using ligand-binding assays alone.
Protein degraders such as PROTACs act catalytically and often show low systemic exposure with strong pharmacodynamic effects. Unlike ADCs, protein degraders require bioanalytical strategies that focus on exposure–response relationships, target protein degradation, and biomarker analysis rather than only plasma concentration measurements.
Key challenges include ultra-low analyte concentrations, non-linear PK-PD relationships, metabolite identification, and linking drug exposure to target protein degradation. These challenges often require integrated PK/PD and biomarker-based bioanalysis rather than traditional PK methods alone.
Hybrid LBA–LC-MS methods combine the sensitivity of immunoassays with the specificity of mass spectrometry. These methods are increasingly used in Antibody Drug Conjugate bioanalytical services to selectively capture ADCs while accurately quantifying payloads and minimizing cross-reactivity.
Regulatory agencies expect well-validated, fit-for-purpose bioanalytical methods with demonstrated selectivity, accuracy, precision, and stability. ADC programs often require multiple validated assays, and regulators closely review how different analytes (intact ADC, total antibody, free payload) are measured and interpreted.
Reference
- Chetana Rao, Vangipuram S Rangan &Shrikant Deshpande.Challenges in Antibody–Drug Conjugate Discovery: A Bioconjugation and Analytical Perspective.https://www.tandfonline.com/doi/full/10.4155/bio.15.81
- Ola M Saad, Ben-Quan Shen, Keyang Xu, S Cyrus Khojasteh, Sandhya Girish &Surinder Kaur.Bioanalytical Approaches for Characterizing Catabolism of Antibody–Drug Conjugates.https://www.tandfonline.com/doi/abs/10.4155/bio.15.87
- Bioanalytical Methods and Strategic Perspectives Addressing the Rising Complexity of Novel Bioconjugates and Delivery Routes for Biotherapeutics.https://pmc.ncbi.nlm.nih.gov/articles/PMC8972746/
- Surinder Kaur.Bioanalysis Special Focus Issue on Antibody–Drug Conjugates.https://www.tandfonline.com/doi/full/10.4155/bio.13.68
- Bioanalytical methods for therapeutic monoclonal antibodies and antibody–drug conjugates: A review of recent advances and future perspectives.https://www.sciencedirect.com/science/article/abs/pii/S0731708519319302
- Challenges in Developing Bioanalytical Assays for Characterization of Antibody–Drug Conjugates.https://www.tandfonline.com/doi/abs/10.4155/bio.11.30

