Introduction
Custom bioanalytical method development and validation are essential for accurate, reproducible, and regulatory-compliant measurement of drugs, metabolites, and biomarkers in biological matrices. At ResolveMass Laboratories, we specialize in designing tailored bioanalytical strategies as part of our comprehensive bioanalytical services to meet the unique requirements of each client’s project, ensuring robust data and regulatory readiness from preclinical to clinical stages. Our expertise spans early discovery through regulated clinical bioanalysis, supporting global drug development programs.
Summary
- ResolveMass Laboratories offers specialized bioanalytical method development and validation services as part of its end-to-end bioanalytical services overview.
- Our services ensure regulatory compliance, data integrity, and reproducibility for PK and PD bioanalysis, toxicokinetic bioanalysis, and advanced biomarker bioanalytical services.
- We provide fully customized analytical solutions using advanced LC–MS/MS bioanalysis of xenobiotics and high-throughput platforms.
- Our experienced scientists deliver accurate, precise, and reliable results supporting IND and NDA bioanalytical submissions.
- Flexible engagement models support biotech startups through large pharmaceutical companies, including affordable bioanalytical services for biotech startups.
1: What is Bioanalytical Method Development?
Bioanalytical method development is the process of creating a reliable, sensitive, and selective analytical method to detect and quantify analytes in biological samples, forming the foundation of regulated and discovery bioanalysis.
At ResolveMass, this process aligns with our specialized bioanalytical method development services and addresses common challenges in bioanalytical method development.
Key Highlights:
- Focuses on Designing for advanced platforms such as LC–MS/MS and HPLC, including high-throughput bioanalysis.
- Ensures sensitivity to detect low concentrations of drugs or metabolites.
- Optimized to manage bioanalytical matrix effects across complex biological matrices.
- Applicable to both small molecule and large molecule bioanalysis, including peptides and biologics.
| Stage | Objective | Benefit |
|---|---|---|
| Sample Preparation | Extract analyte from biological matrix | Minimizes interference |
| Analytical Method Setup | Optimize chromatographic and detection conditions | Accurate quantitation |
| Method Optimization | Adjust parameters for sensitivity & precision | Reliable and reproducible results |
2: What is Bioanalytical Method Validation?
Bioanalytical method validation confirms that a developed method consistently produces accurate and reliable results suitable for regulatory submission and clinical decision-making.
ResolveMass performs validation in accordance with FDA, EMA, and ICH M10 guidelines through its dedicated bioanalytical method validation services and fully regulated bioanalytical services.
Primary Objectives:
- Verify accuracy, precision, selectivity, sensitivity, reproducibility, and stability.
- Ensure compliance with regulatory guidelines like FDA, EMA, ICH M10, and GLP standards.
- Support compliant data generation for clinical bioanalytical services and GLP programs.
- Provide traceable documentation for audits and submissions.
Core Validation Parameters:
- Accuracy: Measures closeness of observed value to true value.
- Precision: Ensures reproducibility across multiple runs.
- Selectivity: Confirms analyte measurement is not affected by other substances.
- Sensitivity: Determines the method’s ability to detect low-level analytes.
- Stability: Confirms analyte remains stable during sample storage and analysis.
These validations directly support GLP bioanalytical services and regulated clinical studies.
3: Why Choose ResolveMass for Bioanalytical Method Development and Validation?
ResolveMass Laboratories stands out as a trusted partner for bioanalytical method development and validation because of our experience, technology, and commitment to regulatory excellence.
Our reputation as a leading bioanalytical CRO is built on scientific rigor, compliance expertise, and client-focused execution.
Key Advantages:
- Experienced Scientists: Deep expertise across drug discovery bioanalysis and late-phase development
- Regulatory Compliance: Supports global programs in North America via bioanalytical services in North America
- Advanced Technology: LC–MS/MS for large molecule bioanalysis and LC–MS for large molecules
- Customized Solutions: Covers cell and gene therapy bioanalysis and biosimilar bioanalysis
- Audit-Ready Documentation: Full traceability and regulatory confidence
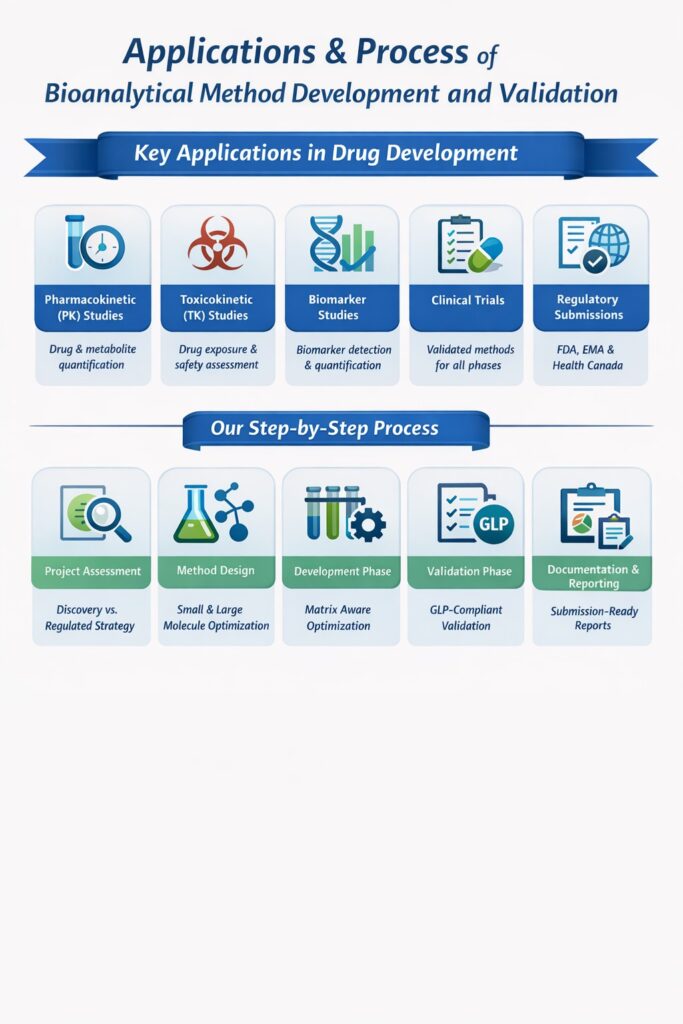
4: Applications of Bioanalytical Method Development and Validation
Our services support a wide range of applications in drug development:
- Pharmacokinetic (PK) Studies: Quantification of drugs and metabolites over time. Integrated with bioanalytical CRO services for PK and TK
- Toxicokinetic (TK) Studies: Safety evaluation by measuring drug exposure. Via specialized toxicokinetic bioanalysis
- Biomarker Studies: Detection and quantification of biological markers for disease or therapeutic response. Advanced biomarker bioanalytical services
- Clinical Trials: Supporting early to late-phase studies with validated bioanalytical methods. End-to-end bioanalytical services in drug development
- Regulatory Submissions: Data generated meets global regulatory standards for FDA, EMA, and Health Canada submissions.
Our Process: Step-by-Step Approach
- Project Assessment: Discovery vs regulated strategy aligned with discovery vs regulated bioanalysis
- Method Design: Optimized for bioanalytical quantification of small and large molecules
- Development Phase: Matrix-aware optimization for precision and robustness
- Validation Phase: GLP-compliant execution under regulated conditions
- Documentation & Reporting: Submission-ready reports supporting regulatory inspections
5: Benefits of Outsourcing Bioanalytical Method Development and Validation
Outsourcing to ResolveMass through our bioanalytical outsourcing and bioanalytical services outsourcing for pharma model provides:
- Reduced operational costs by avoiding in-house infrastructure.
- Access to specialized expertise and technology.
- Faster turnaround times without compromising quality.
- Compliance assurance with global regulatory requirements.
- Transparent planning aligned with bioanalytical testing services cost
Comparison Table: In-House vs Outsourced Services
| Feature | In-House Lab | Outsourced to ResolveMass |
|---|---|---|
| Equipment Costs | High | No upfront cost |
| Expertise | Limited to existing staff | Access to specialized scientists |
| Regulatory Compliance | Requires training | Guaranteed compliance |
| Turnaround Time | Longer | Faster and scalable |
| Documentation & Audit Support | Moderate | Comprehensive, audit-ready |
Conclusion
Custom bioanalytical method development and validation at ResolveMass Laboratories ensures precise, reliable, and regulatory-compliant bioanalytical results across discovery, preclinical, and clinical development. Backed by our full spectrum of bioanalytical laboratory services and CRO expertise, we help sponsors accelerate timelines while maintaining the highest data integrity standards.
Partner with ResolveMass to strengthen your bioanalytical strategy and advance your drug development program with confidence.
Frequently Asked Questions:
Bioanalytical method development and validation is the process of designing, optimizing, and verifying analytical methods used to accurately measure drugs, metabolites, and biomarkers in biological matrices. It ensures reliable, reproducible, and regulatory-compliant data generation for drug development and clinical studies.
Bioanalytical method development and validation are critical because regulatory agencies rely on these data to evaluate drug safety, efficacy, and exposure. Validated methods ensure data integrity across preclinical, clinical, and regulatory submission stages.
Learn more about why bioanalysis is important.
Bioanalytical method validation is governed by FDA, EMA, and ICH M10 guidelines, along with GLP requirements for regulated studies. ResolveMass provides fully compliant regulated bioanalytical services to support global submissions.
Validated bioanalytical methods are required for:
-Pharmacokinetic (PK) studies
-Toxicokinetic (TK) studies
-Clinical trials
-Biomarker and biosimilar studies
These studies are supported through our clinical bioanalytical services and bioanalytical CRO services for PK and TK.
Reference
- Bioanalytical Method Development and Validation: from the USFDA 2001 to the USFDA 2018 Guidance for Industry.https://www.researchgate.net/publication/326495876_Bioanalytical_Method_Development_and_Validation_from_the_USFDA_2001_to_the_USFDA_2018_Guidance_for_Industry
- Bioanalytical method development and validation: Critical concepts and strategies.https://www.sciencedirect.com/science/article/abs/pii/S1570023216308881
- A REVIEW ON BIOANALYTICAL METHOD DEVELOPMENT AND VALIDATION.https://www.researchgate.net/profile/Lokesh-Tijare/publication/316004889_A_review_on_bioanalytical_method_development_and_validation/links/5d8c5276a6fdcc25549a54b5/A-review-on-bioanalytical-method-development-and-validation.pdf
- The history of bioanalytical method validation and regulation: Evolution of a guidance document on bioanalytical methods validation.https://pmc.ncbi.nlm.nih.gov/articles/PMC2751303/
- Bioanalytical Method Validation Guidance Language and a Decade of Progress.https://www.tandfonline.com/doi/full/10.4155/bio-2019-0051
- A Biomarker Assay Validation Approach Tailored to The Context of Use and Bioanalytical Platform.https://www.tandfonline.com/doi/full/10.4155/bio-2023-0110

