Introduction
Scaling bioanalytical capabilities has traditionally meant heavy capital investment in LC-MS labs, specialized personnel, and ongoing maintenance. However, modern drug development teams are increasingly realizing that working with a Bioanalytical Outsourcing CRO allows them to scale bioanalytical support efficiently without the financial and operational burden of building in-house infrastructure.
As bioanalysis becomes more complex across small molecule, large molecule, and biomarker programs, organizations increasingly rely on external expertise in bioanalytical services for drug development.
For emerging biotechs, virtual companies, and even mid-size pharma, outsourcing bioanalysis is no longer a tactical choice — it is a strategic enabler of growth, speed, and compliance.
Summary
- Scaling bioanalysis does not require building or expanding in-house LC-MS laboratories
- Partnering with a Bioanalytical Outsourcing CRO enables rapid capacity expansion without capital expenditure
- Outsourced bioanalysis reduces operational risk, accelerates timelines, and improves regulatory readiness
- Virtual and lean biotech teams can maintain scientific control while avoiding infrastructure burden
- ResolveMass Laboratories Inc. provides cost-effective bioanalytical services for biotech startups
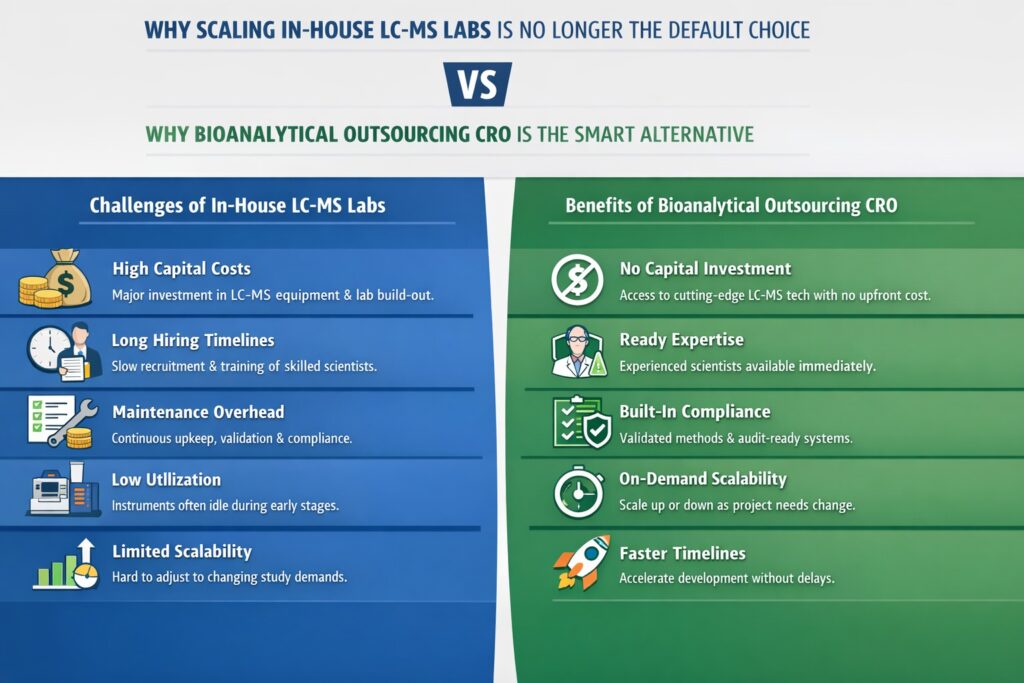
1: Why Scaling In-House LC-MS Labs Is No Longer the Default Choice
Scaling in-house LC-MS laboratories is no longer the default approach because it introduces high financial risk, operational rigidity, and long execution timelines that conflict with the fast, milestone-driven nature of modern drug development. What once signaled control and capability now often becomes a bottleneck.
Modern programs increasingly span PK, TK, biomarker, and clinical bioanalysis, all of which demand flexible and regulated capabilities
👉 https://resolvemass.ca/bioanalytical-services/
Establishing or expanding an internal LC-MS lab requires significant upfront capital investment, including instruments, laboratory space, IT systems, and compliance infrastructure. Beyond cost, the time required to make these labs operational—often 6 to 18 months—can delay critical development milestones.
Key challenges of in-house scaling include:
- High capital expenditure
Triple quadrupole and high-resolution LC-MS systems, laboratory build-outs, service contracts, and software licenses can require millions in upfront spending before generating data.
Advanced LC-MS/MS platforms used for xenobiotic and biomolecule quantification require large upfront investment. - Long timelines for hiring and training
Recruiting experienced bioanalytical scientists is increasingly competitive. Training new staff to align with internal methods, quality systems, and regulatory expectations adds further delay. - Maintenance, validation, and compliance overhead
Instrument qualification, method validation, system suitability testing, and audit readiness require continuous internal effort and specialized expertise.
In-house teams must manage bioanalytical method development and validation continuously. - Underutilization during early or variable pipeline stages
Many early-stage programs do not generate consistent sample volumes, resulting in expensive equipment sitting idle. - Limited flexibility to scale up or down
In-house labs struggle to adapt quickly to fluctuating study demands, often leading to either capacity constraints or wasted resources.
In contrast, a Bioanalytical Outsourcing CRO offers immediate, flexible access to validated LC-MS capabilities without locking organizations into fixed infrastructure costs, enabling development teams to scale only when and where needed.
2: What Does “Scaling Bioanalytical Support” Actually Mean?
Scaling bioanalytical support means expanding analytical capacity, scientific expertise, and operational throughput while maintaining data integrity and regulatory compliance. Importantly, scaling is not just about handling more samples—it’s about supporting more complex programs efficiently.
True bioanalytical scalability includes the ability to:
- Support multiple programs simultaneously
As pipelines grow, teams must analyze diverse molecules, matrices, and study designs in parallel without resource conflicts. - Transition smoothly from discovery to IND-enabling studies
Methods developed in early research must be refined, validated, and scaled without disrupting timelines. - Handle increasing sample volumes without compromising quality
Larger toxicology and clinical studies demand higher throughput while maintaining accuracy, precision, and reproducibility. - Meet evolving regulatory expectations
Requirements around method validation, data traceability, and audit readiness increase with development stage.
This includes support for:
- Discovery vs regulated bioanalysis workflows
- Small and large molecule quantification
- PK, TK, and PD bioanalysis
A specialized Bioanalytical Outsourcing CRO enables this scalability by aligning analytical resources directly with program needs—allowing sponsors to grow scientifically without expanding physical infrastructure.
3: How a Bioanalytical Outsourcing CRO Enables Capital-Free Scaling
A Bioanalytical Outsourcing CRO enables capital-free scaling by providing ready-to-use LC-MS infrastructure, validated workflows, and experienced scientific teams from day one.
Instead of investing in lab expansion, sponsors gain access to:
- Established LC-MS platforms optimized for bioanalysis
CROs operate multiple validated systems capable of handling diverse analytes, matrices, and sensitivity requirements. - Proven assay development and validation workflows
Methods are developed with scalability and regulatory expectations in mind, reducing rework during later phases. - Integrated quality systems
SOPs, documentation, and data integrity practices are already in place and audit-ready. - Senior scientific oversight without staffing overhead
Sponsors benefit from experienced bioanalytical scientists without the burden of permanent hires.
Sponsors gain access to:
- High-throughput bioanalysis platforms
- Regulated bioanalytical services aligned with GLP expectations
- Bioanalytical method validation expertise
This outsourcing model converts fixed capital costs into predictable operational expenses, improving cash flow management, budget forecasting, and investor confidence.
4: Financial Comparison: In-House LC-MS vs Bioanalytical Outsourcing CRO
| Cost Factor | In-House LC-MS Lab | Bioanalytical Outsourcing CRO |
|---|---|---|
| Capital Investment | Very High | None |
| Time to Operational Readiness | 6–18 months | Immediate |
| Staffing Costs | Ongoing fixed costs | Included |
| Equipment Maintenance | Sponsor responsibility | CRO responsibility |
| Scalability | Limited and rigid | High and flexible |
| Regulatory Readiness | Internal burden | Built-in |
For cost-sensitive programs, outsourcing also improves bioanalytical testing services cost predictability
This financial efficiency explains why lean and growth-stage teams increasingly choose a Bioanalytical Outsourcing CRO early, rather than retrofitting outsourcing later.
5: Maintaining Scientific Control While Outsourcing Bioanalysis
A common misconception is that outsourcing bioanalysis means losing scientific control. In practice, the right Bioanalytical Outsourcing CRO operates as an extension of the sponsor’s scientific team, not a black-box vendor.
Effective outsourcing does not mean loss of control. The right Bioanalytical CRO integrates scientific governance into every stage:
- Transparent communication and governance
Clear study plans, data review cycles, and escalation pathways ensure alignment. - Sponsor involvement in assay strategy
Key decisions around method design, sensitivity, and validation are collaborative. - Real-time data access and interpretation
Sponsors engage directly with scientists analyzing and interpreting results. - Direct access to senior bioanalytical experts
Strategic decisions are guided by experienced scientists, not just project managers.
ResolveMass applies this model across virtual biotech programs.
6: Supporting Early-Stage and Virtual Biotech Teams
Virtual biotech companies benefit from outsourced bioanalysis for drug development without infrastructure risk.
Early-stage and virtual biotech companies often lack the scale or predictability to justify internal LC-MS labs. A Bioanalytical Outsourcing CRO model aligns perfectly with their operating realities.
Key advantages include:
- No delays caused by lab construction or equipment procurement
- Ability to run multiple studies with minimal internal headcount
- Reduced investor risk from capital-intensive infrastructure
- Faster progression from proof-of-concept to IND milestones
Outsourcing bioanalysis allows leadership teams to focus on scientific strategy, capital raising, and portfolio decisions, rather than operational logistics.
7: Regulatory Readiness Without Building Quality Systems from Scratch
Regulatory-ready bioanalysis requires validated methods, traceable data, and compliant documentation. Developing this internally demands time, expertise, and continuous maintenance.
A reputable Bioanalytical Outsourcing CRO already provides:
- Established SOPs and quality management systems
- Experience supporting regulatory submissions
- Audit-ready documentation and data traceability
- Deep understanding of global bioanalytical validation expectations
- IND and NDA bioanalytical services
- Clinical bioanalytical services
- Toxicokinetic bioanalysis
This built-in readiness significantly reduces regulatory risk while accelerating development timelines.
8: Scaling Across Drug Development Phases
A strong Bioanalytical Outsourcing CRO supports seamless transitions across all development stages, including:
- Discovery and lead optimization
- Preclinical PK and toxicokinetic studies
- IND-enabling bioanalysis
- Outsourced bioanalysis supports:
- Large molecule bioanalysis
👉 https://resolvemass.ca/large-molecule-bioanalysis/
👉 https://resolvemass.ca/lc-ms-for-large-molecules/ - Biomarker and biosimilar bioanalysis
- Early clinical trials
Because infrastructure, expertise, and quality systems are already in place, sponsors can scale without disruption—even as sample volumes, matrices, and regulatory scrutiny increase.
9: Why Strategic CRO Selection Matters More Than Ever
Not all outsourcing models deliver the same value. Selecting the right Bioanalytical Outsourcing CRO is a strategic decision that directly impacts development success.
Key qualities to evaluate include:
- Demonstrated LC-MS bioanalytical expertise
- Active involvement of senior scientists
- Transparent communication and flexible engagement models
- Proven experience supporting lean and virtual teams
Choosing the right partner means selecting a CRO with:
- Proven bioanalytical laboratory services
- Experience across North American regulatory expectations
- A holistic bioanalytical services overview
ResolveMass Laboratories Inc. positions itself as a strategic bioanalytical partner, focused on long-term scientific and operational success rather than transactional execution.
10: The Future of Bioanalysis: Flexible, Virtual, and Scalable
The industry is shifting away from asset-heavy, fixed infrastructure models toward flexible, expertise-driven bioanalytical partnerships. A Bioanalytical Outsourcing CRO enables organizations to scale at the pace of innovation—without tying growth to lab ownership.
The future favors virtual bioanalytical strategies over asset-heavy labs.
This evolution is redefining how modern drug development teams operate, allocate capital, and compete.
Conclusion
Scaling bioanalytical support no longer requires capital investment in LC-MS laboratories. By partnering with a trusted Bioanalytical Outsourcing CRO, organizations gain access to world-class bioanalysis, maintain scientific rigor, and scale efficiently across development phases.
ResolveMass Laboratories Inc. empowers biotech and pharma teams to move faster, reduce risk, and focus on what matters most—advancing high-quality science toward patients.
Frequently Asked Questions:
Companies outsource LC-MS bioanalysis to avoid high capital investment, long setup timelines, and ongoing operational costs. Outsourcing enables faster study execution, flexible scaling, and access to experienced bioanalytical scientists without maintaining permanent infrastructure.
Yes. Outsourcing bioanalysis is especially beneficial for early-stage and virtual biotech companies because it reduces financial risk, accelerates timelines, and supports milestone-driven development without requiring internal LC-MS labs or large analytical teams.
No. When done correctly, outsourcing bioanalysis does not reduce scientific control. A high-quality Bioanalytical Outsourcing CRO operates as an extension of the sponsor’s team, offering transparent communication, collaborative assay strategy, and direct access to senior scientists.
Bioanalytical services commonly outsourced include PK and TK bioanalysis, biomarker analysis, small and large molecule quantification, method development and validation, regulated GLP bioanalysis, and clinical bioanalytical services.
Yes. Many specialized CROs support both small and large molecule bioanalysis, including peptides, proteins, antibodies, and complex biologics, using fit-for-purpose LC-MS and ligand-binding assay strategies.
Most Bioanalytical Outsourcing CROs can initiate projects immediately or within weeks, as they already have validated LC-MS systems, trained staff, and quality systems in place—significantly faster than building internal capabilities.
Sponsors should evaluate scientific expertise, regulatory experience, quality systems, transparency, communication practices, flexibility, and experience supporting similar development stages or molecule types.
Reference
- Wenkui Li, Jim Glick, Panos Hatsis, Yunlin Fu, Harvey Chin, Paul Moench.An Integrated Outsourcing Practice of Nonclinical LC–MS Bioanalysis and Toxicokinetics at Novartis small Molecule Drug Development.https://www.tandfonline.com/doi/abs/10.4155/bio-2021-0072
- Yan Song, Raj Dhodda, Jun Zhang & Jens Sydor.A High Efficiency, High Quality and Low Cost Internal Regulated Bioanalytical Laboratory to Support Drug Development Needs.https://www.tandfonline.com/doi/abs/10.4155/bio.14.104
- Nico van de Merbel, Mark Jean Gnoth, Amanda Wilson, Peter Blattmann, Benno Ingelse, Gregor Jordan.Perspective on LC-MS(/MS) for biotherapeutic and biomarker proteins in research and regulated Bioanalysis: a consolidation of more than a decade of experience across the European Bioanalysis Forum community.https://www.tandfonline.com/doi/full/10.1080/17576180.2024.2418251
- Anne-Françoise Aubry.LC–MS/MS Bioanalytical Challenge: Ultra-High Sensitivity Assays.https://www.tandfonline.com/doi/abs/10.4155/bio.11.166
- Naiyu Zheng, Hao Jiang & Jianing Zeng.Current Advances and Strategies Towards Fully Automated Sample Preparation for Regulated LC–MS/MS Bioanalysis.https://www.tandfonline.com/doi/abs/10.4155/bio.14.161
- Principles and Applications of LC-MS/MS for the Quantitative Bioanalysis of Analytes in Various Biological Samples.https://d1wqtxts1xzle7.cloudfront.net/53516784/Tandem_Mass_Spectrometry_-Applications_and_Principles.pdf?1738363831=&response-content-disposition=inline%3B+filename%3DTANDEM_MASS_SPECTROMETRY_APPLICATIONS_AN.pdf&Expires=1769179481&Signature=DuOLjU6Z~x2EZpUV5Q7Fh3egwrbpehYGqFUTLygUaMldCbcQX9WRcs-wg1DuKv2h5CpYgTnO7SXZH6aLXMycKWEQAMQXEcW3ft07zlkAZEl4zIpMgx9vyRnzwLk-dXHw3eAeUxJ1kq2JBKv4AQ~mxgdOoFEOcMAzcA1AL3COzvWfoUuVNzg2yOgw0W6s2tGU4SR-quj~MI-UFhfLzZexkbv30rwAam4O7riYwoHJrqLn1J19SFOTNT0Y~sUWBIlpcX3B~WtCDjX~Y8pPT8AsaNiRP4EX6yiZtG5tMT21qV0d5gCv~KXRKALykd0yqWHawck49DtRMktoCKCSA8Q-Rg_&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA#page=471

