✅ Summary: What You’ll Learn
- The exact role of CMC services for IND and NDA submissions
- Why CMC is the #1 cause of regulatory delays (and how to avoid them)
- Key differences between CMC requirements for IND vs. NDA
- How to build a regulatory-aligned CMC strategy
- Common pitfalls in CMC dossiers and how to prevent them
- Detailed CMC timeline aligned with FDA expectations
- What FDA reviewers look for in your CMC sections
- Importance of data integrity, comparability, and scalability
- Real-world checklists for analytical, process, and stability data
- How experienced CMC partners reduce submission risk
🧪 Introduction: Why CMC Services for IND and NDA Matter
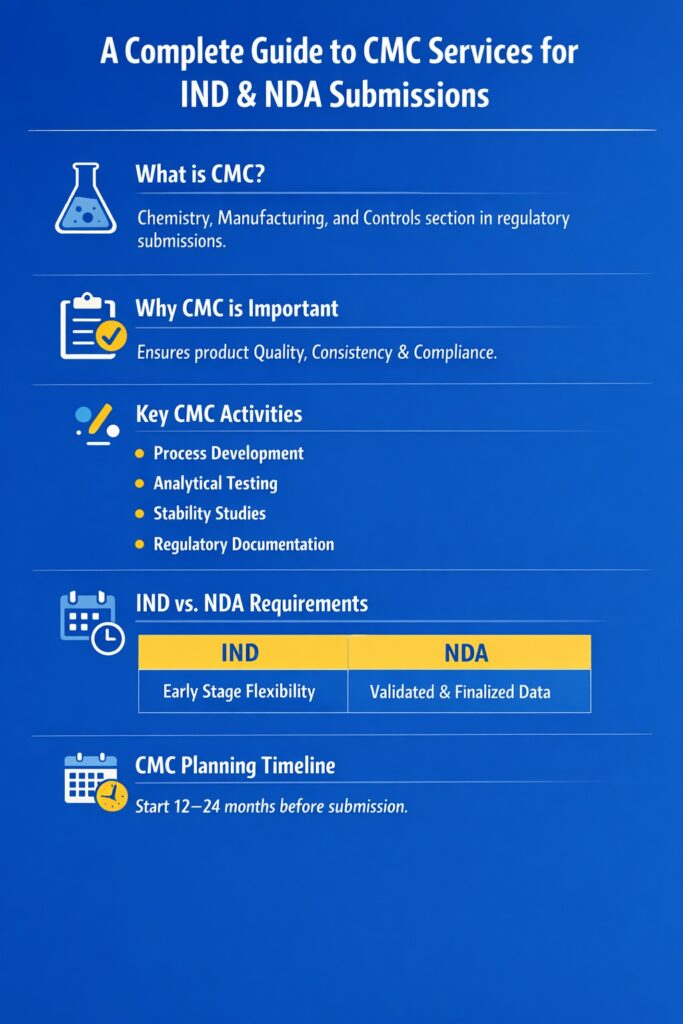
CMC services for IND and NDA play a critical role in the drug development process. These services influence timelines, FDA review outcomes, and your product’s ability to progress from trials to market. Even strong drug candidates can face regulatory delays without a solid CMC strategy.
Whether you’re preparing for a pre-IND meeting or assembling a full NDA package, the CMC (Chemistry, Manufacturing, and Controls) section is often the deciding factor in regulatory progression. Agencies depend on CMC data to confirm product safety, consistency, and quality. Having a clear, complete, and compliant CMC package can build trust with reviewers and reduce delays.
This guide provides a thorough and practical roadmap to developing a regulatory-aligned CMC approach that drives successful IND and NDA submissions.
Streamline Your Path to Market Early-stage planning is the foundation of clinical success. Discover how our specialized Drug Discovery Services can help you build a robust scientific framework from the very beginning.
🧪 Why Are CMC Services for IND and NDA Submissions So Important?
The CMC section is one of the most detailed and closely reviewed parts of both IND and NDA submissions. It shows that the drug substance and product can be made consistently and meet quality standards.
Proper CMC documentation proves that manufacturing is well-controlled and scientifically supported. It also confirms that formulation choices and excipients won’t compromise product stability or performance. Without strong data, regulators may stop a product from moving forward.
CMC services for IND and NDA bridge research and commercial manufacturing, making sure that data stays relevant throughout development. This consistent approach helps avoid rework, unexpected questions, and costly setbacks.
Key Roles of CMC in Regulatory Submissions
- Define drug substance and product specifications
- Prove process control and consistency across batches
- Justify formulation design and excipient choices
- Provide long-term stability to support shelf life
- Detail packaging systems and labeling controls
- Ensure full traceability and compliance with 21 CFR Part 11
Ensure Methodological Excellence Regulators require precise, reproducible data at every stage. Explore our Analytical Method Development and Validation Services to ensure your testing protocols meet global compliance standards.
Differences in CMC Services for IND and NDA Submissions
CMC expectations change significantly between IND and NDA stages. In early phases (IND), the focus is on product safety for human testing. In later stages (NDA), it’s about commercial-scale production with consistent quality.
IND development allows more flexibility, but for NDA, all methods must be validated and manufacturing processes finalized. Poor planning early on can lead to bigger problems later.
A forward-looking approach to CMC services for IND and NDA ensures smooth progression by anticipating commercial and regulatory needs.
| Component | IND Stage (Phase 1/2) | NDA Stage (Pre-Approval) |
|---|---|---|
| Manufacturing Process | Developmental, small scale | Commercial scale, validated |
| Analytical Methods | Semi-quantitative allowed | Fully validated, ICH-compliant |
| Stability Data | Accelerated accepted | Long-term, ICH Q1A(R2) compliant |
| Impurity Profiling | Basic accepted | Full degradation/genotoxic data |
| Process Validation | Not required | Mandatory for commercial scale |
| Specifications | Broad accepted | Tight, reproducible, justified |
🔍 What’s Included in Full CMC Services for IND and NDA?
A complete CMC suite includes strategic planning, technical development, and regulatory documentation. These elements must align to ensure consistency and compliance.
Fragmented or late CMC planning often causes gaps and delays. A unified strategy supports better quality and faster submissions.
Strategic CMC Planning
- Clear IND to NDA transition timelines
- Risk-based control strategies
- Pre-IND and pre-NDA meeting support
Technical CMC Execution
- Drug substance and product development
- Method development, validation, and qualification
- Container-closure and extractable/leachable studies
- GMP-compliant batch production
Regulatory CMC Documentation
- CTD Module 3 (Quality) authoring and review
- CMC gap analysis and resolution
- Timely response to FDA Information Requests
These combined efforts ensure your CMC services for IND and NDA meet all FDA expectations.
Protect Your Product from Packaging Risks Interaction between your drug and its container can compromise safety. Use our Extractables and Leachables Testing to verify the integrity of your packaging systems.
📅 When to Begin CMC Planning?
Planning should start early—ideally 12 months before IND submission and 18–24 months before NDA. This timeline allows time for method validation, stability studies, and manufacturing readiness.
Early planning also improves communication between teams, ensuring that data is consistent and submission-ready.
| Timeline (Before Submission) | CMC Milestones |
|---|---|
| 18–24 Months | Process development and route selection |
| 12 Months | Analytical method validation starts |
| 9 Months | Begin stability and packaging evaluations |
| 6 Months | Draft Module 3 and compile data |
| 3 Months | Internal QA and final CMC review |
Proactively Identify Degradation Pathways Understanding how your molecule breaks down is vital for long-term success. Learn how our Forced Degradation Studies provide the predictive data needed for robust shelf-life claims.
❌ Common Mistakes in CMC Submissions (And How to Prevent Them)
Many delays occur due to poor planning and missing documentation—not bad science. Even small issues can raise red flags for regulators.
Problems often include unvalidated methods, unclear impurity data, and inconsistent stability results. Fixing these late in development is expensive and risky.
Thorough CMC services for IND and NDA help catch these problems early.
Top 5 Common CMC Mistakes
- Analytical methods not validated
- Impurities or degradation data incomplete
- Poor scale-up documentation
- Missing or inconsistent stability results
- Weak control strategies not based on QbD
How CMC Services Help
- Conduct GMP audits and QA checks
- Create traceable, audit-ready data packages
- Build control strategies aligned with FDA guidelines
Meet Modern Purity Standards Nitrosamine impurities are a major focus for current FDA and EMA inspectors. Secure your submission with our high-sensitivity Nitrosamine Analysis to ensure your product is contaminant-free.
🔎 What FDA Reviewers Look for in CMC Sections
FDA reviewers check if the proposed control strategy manages product risks effectively. Safety and consistency are key.
They want clearly written manufacturing processes, validated test methods, and justification for every key decision.
Well-prepared CMC services for IND and NDA help answer these questions upfront.
FDA Focus Areas
- Reliable synthesis and process consistency
- Robust and validated analytical methods
- Suitable container-closure systems
- Shelf life supported by stability data
- Specifications tied to clinical and production risks
🔁 Ensuring Data Integrity and Traceability
Regulators expect reliable, auditable CMC data that follows ALCOA+ principles. Any inconsistencies can trigger compliance concerns.
Data systems must be secure, traceable, and comply with regulations like FDA 21 CFR Part 11.
ALCOA+ Principles
- Attributable
- Legible
- Contemporaneous
- Original
- Accurate
- Complete, Consistent, Enduring, and Available
Strong governance ensures your CMC services for IND and NDA pass audits and inspections confidently.
🧬 CMC Services in Analytical and Process Validation
Validation proves that your methods and processes work consistently and are suitable for use. It’s a major regulatory requirement.
CMC teams manage these validations to meet FDA and ICH guidelines.
Core Validation Packages
- Process validation using PPQ batches
- Analytical method validation (ICH Q2(R2))
- Cleaning validations and residue limits
- Container and holding time studies
- Justification of in-process controls
🛠️ Why Partnering with CMC Experts Reduces Risk
Experienced partners bring deep regulatory knowledge and technical know-how. They help avoid common errors and ensure quality submissions.
A reliable partner knows regional regulations and offers integrated support across formulation, QA, and compliance.
Qualities of a Strong CMC Partner
- Experience with multiple dosage forms
- Knowledge of FDA, EMA, and Health Canada guidelines
- In-house analytical and QA expertise
- Fast turnaround with strong documentation
- Proven success with IND and NDA submissions
Get Expert Support for Your Next Filing Don’t leave your regulatory success to chance. Contact the ResolveMass team today for a customized consultation on your CMC requirements.
✅ Conclusion: Invest Early in CMC Services for IND and NDA
CMC services for IND and NDA are crucial to product success—not just paperwork. Starting early helps avoid costly delays and builds regulator confidence.
A solid CMC program reduces FDA questions, avoids clinical holds, and makes the transition to commercial scale much smoother.
Whether you’re at the IND or NDA stage, early investment in CMC ensures long-term product success.
📞 Need Expert CMC Services for IND and NDA?
ResolveMass Laboratories Inc. offers full-service, submission-ready CMC services for IND and NDA that help get your product to clinic and market—faster and safer.
FAQs on CMC Services for IND and NDA
CMC services refer to the scientific, manufacturing, and regulatory activities that ensure a drug product is consistently produced with high quality. These services support compliance with FDA guidelines and are essential for both early development (IND) and final approval (NDA) stages.
At the IND stage, the focus is on product safety for early-phase clinical trials, and more flexibility is allowed. For NDA, the requirements are stricter—processes must be validated, methods fully qualified, and commercial readiness clearly demonstrated.
One of the leading causes of delay is incomplete or poorly validated analytical methods. Weak justification of specifications or inconsistencies in stability data can also lead to FDA questions and result in a Complete Response Letter (CRL).
Yes, you can file an IND without all CMC components finalized. However, any missing information must be clearly justified, and a plan should be in place to complete the required studies as development progresses.
The CMC section of the NDA, typically found in Module 3 of the Common Technical Document (CTD), includes detailed information on the drug’s chemistry, manufacturing processes, controls, specifications, and quality assurance. It ensures the product is safe, consistent, and suitable for market approval.
Reference
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/


