🔍 Summary: Key Takeaways
- A well-defined CMC strategy for orphan drugs is crucial due to limited patient populations and accelerated regulatory timelines.
- IND-phase CMC planning should balance early data requirements with flexibility for post-approval commitments.
- For NDA, emphasis should be on robust comparability, product characterization, and long-term stability data.
- Lifecycle management, scalability, and quality-by-design (QbD) principles are pivotal to ensure regulatory success.
- Regulatory agencies offer significant flexibility under orphan drug designation, but scientific justification is mandatory.
- Supply chain robustness and risk mitigation strategies can make or break NDA approval.
- Engage early with the FDA through Type B meetings to align on CMC expectations for orphan drugs.
- Analytical method validation, reference standards, and impurity control must be strategically planned.
- Stability studies, though challenging, are non-negotiable for shelf-life determination.
- CMC compliance is critical for successful market access and long-term product sustainability.
📌 Introduction: Why CMC Strategy for Orphan Drugs Is Crucial
A well-developed CMC strategy for orphan drugs is essential to enable fast-track development while maintaining product quality and safety. Orphan drug programs often face resource constraints and are conducted with less clinical data compared to conventional drug development.
Small patient groups and limited drug supply present unique risks. Addressing these early through risk-based decisions, scientific justification, and strong regulatory communication helps keep the program on track.
Explore our foundational services: Comprehensive Chemistry, Manufacturing, and Controls (CMC) Services
Faster timelines only increase the importance of sound early-stage CMC planning. IND-phase decisions influence NDA readiness and future regulatory commitments. A flexible yet compliant CMC strategy helps maintain continuity from clinical trials to commercialization.
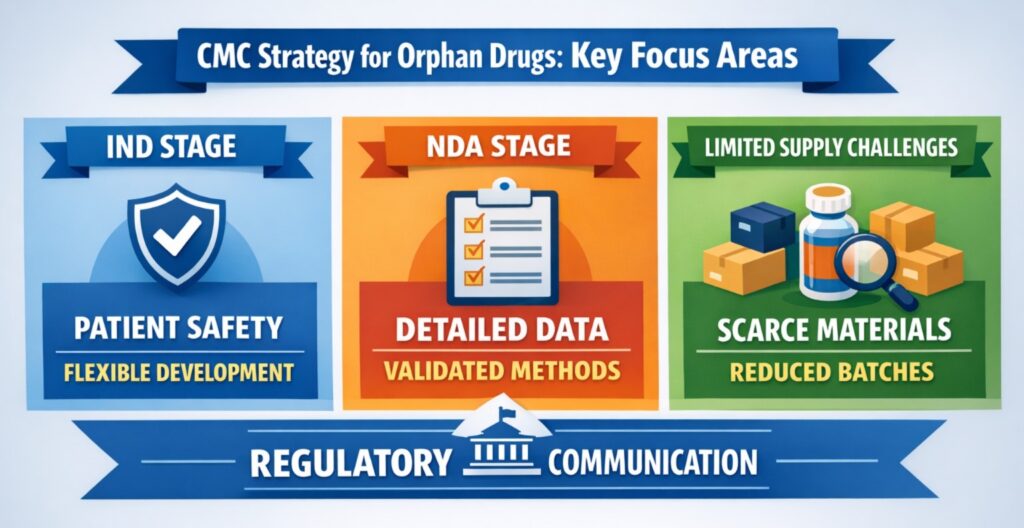
🧪 IND-Phase CMC Strategy for Orphan Drugs
During the IND phase, ensuring patient safety is the top priority, while still allowing room for product and process adjustments. Regulators understand that orphan drug development may follow a less traditional path.
Early efforts should aim at identifying critical quality attributes and establishing essential control strategies. Over-documentation can hinder future process refinement, so the goal is a lean yet compliant CMC approach.
Optimize your early-stage development: Expert CMC CRO Services for Orphan Drugs
✅ Key Considerations at IND Stage
Minimum Viable CMC Package
To begin clinical trials, enough information must be shared with regulators to confirm product safety and manufacturing reliability. This includes the manufacturing process, materials used, and product specifications.
Even with fewer data requirements, impurity profiling and basic product characterization are still expected. Sponsors should document any risks and establish controls to manage them.
Navigate your initial filing: Specialized IND CMC Services
Leverage Early-Phase Flexibility
The FDA often allows a phase-appropriate GMP approach for orphan drugs. This reduces unnecessary burden while ensuring safety. Early identification of critical process parameters helps maintain batch-to-batch consistency.
Sponsors should document known limitations and outline planned process enhancements for future stages.
Focus Areas for IND Submission
| Requirement | IND Expectation for Orphan Drugs |
|---|---|
| Drug Substance | Early synthesis route, impurity controls, identity |
| Drug Product | Formulation rationale, usage-based stability data |
| Manufacturing Facility | GMP compliance statement, facility overview |
| Controls | Analytical methods, batch release criteria |
These components show that the investigational product can be manufactured safely and consistently for clinical use.
Bridging Studies and Scalability
Planning for scalability early helps avoid problems during later stages. Even small scale changes can impact impurities and overall product quality.
Establishing traceability across batches and comparing lab-scale to clinical-scale materials strengthens regulatory confidence. Documenting how the process evolves ensures smoother transitions from IND to NDA.
Plan for the transition: Understanding IND vs NDA CMC Requirements
🔄 Transitioning CMC from IND to NDA for Orphan Drugs
As the development moves from IND to NDA, the focus shifts from flexibility to consistency. The CMC strategy for orphan drugs must now demonstrate reliable and repeatable manufacturing processes.
Although some flexibility remains for orphan drugs, regulators still expect complete, scientifically justified data, especially when changes have been made since the IND stage.
Master the transition strategy: CMC Services for IND and NDA Success
🔍 Core Focus at NDA Stage
Comprehensive Product Characterization
A full impurity profile should be presented, including qualification of acceptable limits and how they were determined. Stability-indicating methods are vital for demonstrating product reliability.
Reference standards must be validated and traceable. Sponsors should clearly explain any differences between IND and NDA batches.
Ensure product safety: Advanced Impurity Profiling in CMC
Validated Analytical Methods
At NDA, analytical methods must be fully validated for accuracy, precision, specificity, linearity, and robustness. Partial validation is no longer acceptable.
Stability-indicating capability must be proven with forced degradation studies. Sponsors should also present a method lifecycle management plan in line with ICH Q2(R2).
Strengthen your data package: Analytical Method Development for IND and NDA
Stability Data Requirements
| Type | Minimum Recommended |
|---|---|
| Long-Term | 12 months (25°C/60%RH) |
| Accelerated | 6 months (40°C/75%RH) |
If full data is unavailable, scientifically valid extrapolation models may be used. Post-approval commitments should outline how ongoing data will be gathered.
Secure your shelf-life data: Rigorous Stability Studies in CMC
Drug Substance Controls
For orphan drugs, the FDA may accept fewer than three commercial-scale batches if the scientific rationale is strong. Process data should demonstrate consistent control of critical quality parameters and impurities.
🛠️ Manufacturing Controls and Quality Risk Management
Orphan drug development often requires non-traditional validation approaches. A risk-based control strategy allows reduced documentation where appropriate while maintaining safety and product quality.
Sponsors should use structured risk assessment tools and ensure controls evolve as process understanding improves.
📌 Critical Implementation Elements
Process Validation
Lifecycle validation across FDA’s three stages (1–3) supports ongoing control. When batch numbers are limited, concurrent validation may be acceptable, provided protocols and success criteria are clearly defined.
Control Strategy Framework
Applying Quality by Design (QbD) allows better control over critical process points and supports regulatory flexibility. Tools like FMEA help evaluate risks in raw materials, excipients, and packaging components.
Batch Release Strategy
Real-time release testing (RTRT) may be considered if robust validation and process control support it. Even in low-volume settings, rigorous documentation and batch testing are required for release.
🧬 Challenges with Limited Supply and Material Constraints
Material limitations are common in orphan drug development. A strategic CMC strategy for orphan drugs must manage these constraints without delaying progress.
Without proper planning, these challenges can impact clinical trials and regulatory submissions.
🎯 Strategic Responses
Bridging Studies
When clinical material is scarce, preclinical lots may support development if comparability is established through analytical data. This maximizes material use while ensuring quality standards.
Reduced Batch Numbers
Fewer validation batches may be accepted by FDA for orphan drugs if supported by strong risk analysis. Detailed documentation must explain any deviation from standard practices.
Reference Standards
Initial reference standards may be used early, but by NDA, they must be fully qualified and traceable to ensure consistent product performance.
📅 CMC Lifecycle Management Post-Approval
CMC responsibilities extend beyond NDA approval. Effective lifecycle planning ensures uninterrupted supply and regulatory compliance. Early planning helps anticipate changes without disrupting operations.
🔄 Key Lifecycle Elements
Change Control Protocols
Sponsors can submit protocols for expected changes in advance. This allows timely implementation of changes such as new manufacturing sites or process improvements.
Ongoing Stability Monitoring
Post-approval stability testing is critical to confirm shelf life and product quality. Sponsors should monitor trends and address deviations quickly.
Technology Transfer Considerations
Proper documentation of process understanding supports future tech transfers or scale-ups. This reduces risks during transitions.
🧑⚕️ Regulatory Engagement: Proactive Communication with FDA
Early dialogue with the FDA is crucial when developing a CMC strategy for orphan drugs. Well-prepared meetings ensure clarity, reduce surprises, and build regulatory trust.
Prepare for agency review: Insights into FDA CMC Review Processes
📝 Regulatory Milestones
- Pre-IND Meeting (Type B): Share early CMC plans and request feedback.
- End-of-Phase 2 Meeting: Confirm data expectations for NDA.
- Pre-NDA Meeting: Ensure final CMC module is complete and identify remaining gaps.
Briefing materials should focus on critical analytical plans, risk controls, and unresolved CMC questions.
🌍 Global Considerations: EU and ROW Submissions
If the drug is intended for international markets, it’s important to align early with regional requirements. A harmonized CMC strategy for orphan drugs supports smoother global submissions.
🌐 Consider:
- EU Requirements: EMA offers flexibility but may request more detailed batch data and comparability evidence.
- WHO Prequalification: Align early if your product is intended for global access or public health programs.
- ICH Guidelines: Following ICH Q8–Q11 ensures international regulatory consistency.
📌 Conclusion: Strategic Excellence Is Mandatory
An optimized CMC strategy for orphan drugs is not just a regulatory necessity—it’s key to timely approval, sustained supply, and market access. Every decision from IND through NDA must be supported by robust science and thoughtful risk management.
ResolveMass Laboratories Inc. offers deep expertise in designing and implementing efficient CMC strategies for orphan drugs, ensuring speed without sacrificing quality.
❓ Top 10 Most Asked FAQs
A CMC strategy at the IND stage ensures the investigational product is safe for initial human use, even with limited data. It allows flexibility in manufacturing and development, while still meeting regulatory expectations for quality and traceability. For orphan drugs, this strategy helps manage early-stage risks effectively.
The FDA provides some regulatory flexibility for orphan drugs due to limited patient numbers and materials. This may include fewer required batches or reduced data, but every adjustment must be supported with clear scientific reasoning. Risk-based approaches are essential to justify any deviations from standard requirements.
While three validation batches are standard, orphan drugs may be approved with fewer batches if strong scientific justifications are provided. The FDA may accept scaled-down data when full-scale production is not feasible. Sponsors must demonstrate consistent process control and product quality.
Early-phase reference standards can only be used for NDA submission if they are fully qualified and supported by data. If not, new reference materials should be developed and validated. Ensuring traceability and consistency is crucial for regulatory acceptance.
Full QbD (Quality by Design) documentation is not mandatory for orphan drugs, but applying QbD principles enhances scientific justification. It also supports better control strategies and regulatory flexibility. Implementing QbD strengthens the overall development and submission package.
Real-time release testing (RTRT) can be applied to orphan drugs if supported by strong process understanding and validation data. However, limited batch availability may make it harder to meet RTRT requirements. Still, it can be valuable for reducing release timelines in small-scale production.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas


