Introduction
Cost-effective bioanalytical services are essential for seed-stage and Series A biotechs because early development decisions are made under tight budgets and aggressive timelines. Every dataset must support scientific confidence, investor scrutiny, and future regulatory progression without unnecessary cost escalation. Bioanalysis plays a foundational role across the drug development lifecycle, from discovery through regulated studies, as outlined in ResolveMass’ overview of bioanalytical services in drug development.
For early-stage biotech companies, bioanalysis is not just a technical requirement—it is a strategic function. Outsourcing cost-effective bioanalytical services to an experienced bioanalytical CRO such as ResolveMass Laboratories Inc. enables companies to move faster, control burn rate, and generate high-quality data that withstands due diligence and regulatory review.
Summary
Early-stage biotech companies face a critical challenge: obtaining high-quality bioanalytical data while managing limited budgets. Cost-effective bioanalytical services enable seed-stage and Series A biotechs to advance their drug development programs without compromising scientific rigor or regulatory compliance.
Key Takeaways:
- Cost-effective bioanalytical services support reliable PK, TK, and biomarker bioanalysis without exhausting capital
- Outsourcing bioanalysis avoids the cost of building in-house infrastructure
- Fit-for-purpose bioanalytical method development and validation reduces rework and cost
- Experienced CRO partnerships minimize scientific and regulatory risk
1: What Are Cost-Effective Bioanalytical Services?
Cost-effective services deliver scientifically robust, reproducible, and regulatory-aligned analytical data while optimizing cost by eliminating unnecessary complexity, over-validation, and inefficient workflows. For early-stage biotech companies, cost-effectiveness is achieved not by compromising quality, but by aligning analytical rigor precisely with the stage and objective of development.
At their core, cost-effective bioanalytical services are designed to support decision-making, not perfection for its own sake. They focus on generating reliable exposure, safety, and biomarker data that enables confident progression—or termination—of a program at the right time.
Key Principles of Cost-Effective Bioanalytical Services
Cost-effective bioanalytical services emphasize the following principles:
- Generating decision-enabling data
Data must answer critical scientific and development questions such as dose proportionality, exposure margins, and safety windows. Assays are designed to inform next steps, not to over-engineer results. Assays focus on critical development questions such as dose proportionality, exposure margins, and safety windows through reliable bioanalytical quantification. - Aligning analytical rigor with development stage
Early discovery and preclinical programs benefit from fit-for-purpose validation rather than full ICH validation. This distinction is explained in discovery vs regulated bioanalysis. - Designing workflows that scale without rework
Methods developed early should be adaptable for IND-enabling and clinical phases, avoiding costly redevelopment later. Early methods should transition smoothly into IND- and NDA-enabling bioanalysis.
This approach ensures early-stage biotechs pay only for what they need now, while remaining strategically prepared for future regulatory and investor milestones.
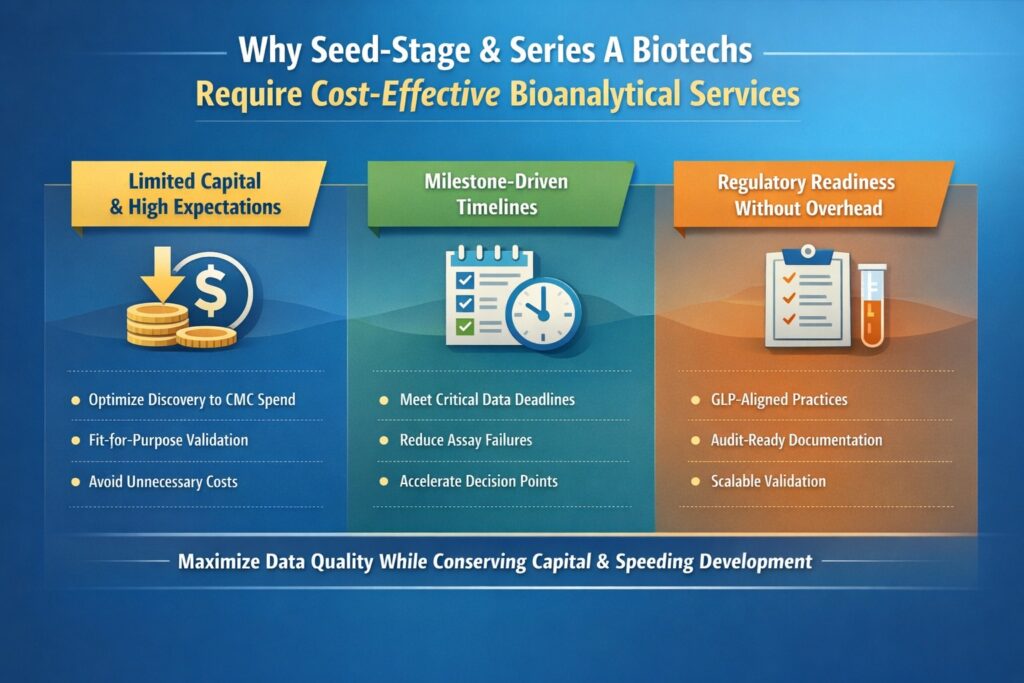
2: Why Seed-Stage and Series A Biotechs Require Cost-Effective Bioanalytical Services
Seed-stage and Series A biotechs operate under constraints that make cost-effective services essential rather than optional. Limited funding, lean teams, and milestone-driven development demand maximum value from every outsourced activity.
2.1 Limited Capital and High Expectations
Early-stage funding must support multiple parallel activities, including discovery optimization, formulation development, toxicology studies, and regulatory planning. Bioanalysis that is over-scoped or inefficient can quickly drain capital without adding proportional value.
Cost-effective services help preserve cash while still producing high-quality data that can withstand internal review, investor scrutiny, and partner due diligence.
Bioanalysis that is over-scoped or poorly planned can rapidly consume capital. Purpose-built, affordable bioanalytical services for biotech startups protect runway while preserving data integrity.
2.2 Milestone-Driven Timelines
Progression decisions, fundraising events, and partnering discussions often depend on timely PK, TK, or biomarker data. Failed assays, reanalysis, or poorly designed studies can delay milestones and negatively impact valuation.
By working with experienced CROs that specialize in early programs, biotechs reduce the risk of delays and data gaps.
2.3 Regulatory Readiness Without Overhead
Even preclinical bioanalysis must follow traceable, auditable, and scientifically justified practices. Cost-effective bioanalytical services ensure compliance with GLP-aligned principles without imposing the cost structure of late-stage clinical bioanalysis. Preclinical studies must still follow traceable and auditable practices aligned with regulated bioanalytical services and GLP expectations.
3: In-House Bioanalysis vs Outsourced Bioanalytical Services
For most seed-stage and Series A companies, outsourcing is the most efficient path to cost-effective bioanalytical services.
| Factor | In-House Bioanalysis | Outsourced Bioanalytical Services |
|---|---|---|
| Initial investment | Very high (equipment, hiring, validation) | Minimal |
| Time to readiness | 12–24 months | Immediate |
| Regulatory burden | Fully internal | CRO-managed |
| Scalability | Limited by staff and instruments | High |
| Cost predictability | Low | High |
Outsourcing provides access to high-throughput bioanalysis, validated LC-MS/MS platforms, and established quality systems without fixed infrastructure costs.
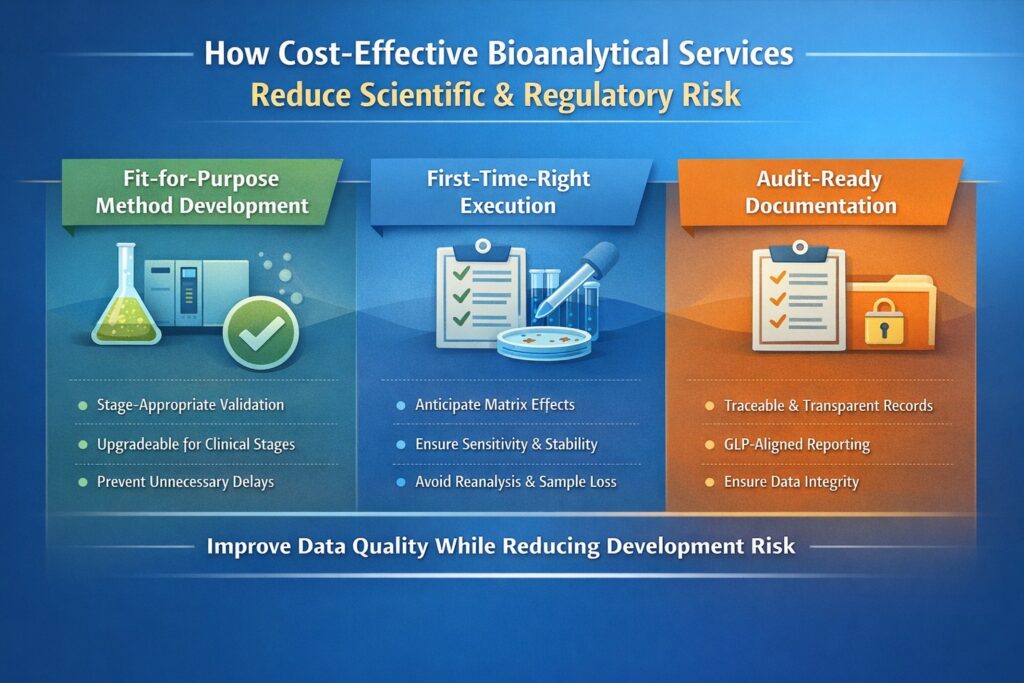
4: How Cost-Effective Bioanalytical Services Reduce Scientific and Regulatory Risk
Cost-effective services do not merely reduce spending; they actively reduce development risk by improving data quality and decision confidence.
4.1 Fit-for-Purpose Method Development
Early-stage studies rarely require full regulatory validation. Experienced CROs design partial or stage-appropriate validation strategies that meet scientific needs while remaining upgradeable for later phases.
This prevents unnecessary delays and avoids repeating method development when programs advance.
Experienced CROs apply stage-appropriate validation strategies supported by robust bioanalytical method development and its challanges frameworks.
4.2 First-Time-Right Execution
Poor assay design leads to missed samples, reanalysis, and inconclusive results—each increasing cost and timeline risk. Expert bioanalytical scientists anticipate challenges such as:
- Matrix effects
- Sensitivity limitations
- Stability issues
- Metabolite interference
Addressing these early ensures robust data generation from the first study.
Early identification of bioanalytical matrix effects, sensitivity limits, and stability challenges prevents reanalysis.
4.3 Audit-Ready Documentation
Even exploratory studies may be reviewed by investors, partners, or regulatory consultants. Proper documentation, traceability, and data integrity protect program credibility without excessive administrative burden.
5: Core Elements of Cost-Effective Bioanalytical Services
True cost efficiency results from deliberate scientific and operational choices rather than simple cost-cutting.
5.1 Optimized LC-MS/MS Platforms
Modern LC-MS/MS technologies enable:
- Shorter run times
- Lower sample volumes
- Multiplexed analyses
- Reduced reanalysis rates
These efficiencies directly lower per-sample costs while maintaining analytical performance.
Advanced LC-MS/MS workflows support xenobiotics, metabolites, and complex modalities.
5.2 Strategic Study Design
Upfront scientific consultation helps avoid common cost drivers such as:
- Excessive sampling time points
- Redundant biological matrices
- Unnecessary repeat analyses
Well-designed studies maximize information yield while minimizing analytical workload.
Careful planning avoids redundant matrices and unnecessary repeat analyses across bioanalytical laboratory services.
5.3 Flexible Validation Approaches
Cost-effective services tailor validation depth to study intent:
- Exploratory PK studies: Partial validation
- IND-enabling studies: Full validation
- Biomarker studies: Customized qualification
This flexibility ensures compliance without over-investment.
Validation strategies scale from exploratory PK to clinical bioanalytical services.
6: Applications of Cost-Effective Bioanalytical Services for Early Biotechs
Cost-effective bioanalytical services support multiple early development applications, including:
- Pharmacokinetic (PK) studies for exposure and dose proportionality
- Toxicokinetic (TK) studies for safety margin evaluation
- Bioavailability and formulation comparisons
- Exploratory biomarker and pharmacodynamic studies
Each application benefits from analytical rigor tailored to development stage and decision requirements.
7: Why ResolveMass Laboratories Inc. Is Trusted by Early-Stage Biotechs
ResolveMass Laboratories Inc. specializes in delivering cost-effective services to virtual, seed-stage, and Series A biotech companies.
Proven Experience
Our scientists bring hands-on experience across small molecules, metabolites, and complex biological matrices, ensuring scientifically sound data generation from early discovery through IND-enabling studies.
Early-Phase Expertise
We actively advise clients on:
- What data is essential today
- What can be safely deferred
- How to design assays that scale to clinical development
Transparent Project Scoping
Clear scopes, predictable pricing, and realistic timelines ensure cost-effective services remain truly cost-effective throughout the project lifecycle.
ResolveMass Laboratories Inc. delivers integrated bioanalytical services across North America with deep expertise in outsourcing models for virtual biotechs.
Our end-to-end capabilities are summarized here:
https://resolvemass.ca/resolvemass-bioanalytical-services-overview/
https://resolvemass.ca/bioanalytical-services/
Conclusion
Cost-effective services give seed-stage and Series A biotechs a decisive competitive advantage. By partnering with an experienced CRO, early-stage companies gain high-quality, compliant, and scalable data without the burden of building internal infrastructure.
ResolveMass Laboratories Inc. enables emerging biotech companies to advance confidently and efficiently through early development using cost-effective bioanalytical services built for long-term success.
Frequently Asked Questions:
Outsourcing eliminates high upfront costs for instruments, personnel, and quality systems. Cost-effective bioanalytical services provided by CROs offer immediate access to expertise, validated platforms, and regulatory-ready processes that are difficult for early-stage companies to build internally.
Cost-effective bioanalytical services support:
-Pharmacokinetic (PK) studies
-Toxicokinetic (TK) studies
-Biomarker and pharmacodynamic studies
-Bioavailability and formulation comparison studies
Each study is designed with analytical rigor appropriate to its development stage.
They reduce risk by ensuring first-time-right execution, anticipating matrix effects and sensitivity challenges, and producing audit-ready data. This minimizes reanalysis, delays, and regulatory setbacks that can negatively impact funding and timelines.
Yes. Cost-effective bioanalytical services support small molecules, large molecules, biosimilars, and advanced modalities using LC-MS/MS and ligand-binding assays, with workflows designed to scale as programs advance.
Early-stage biotechs should look for:
-Proven early-phase experience
-Fit-for-purpose validation strategies
-Transparent pricing and scoping
-Scalable methods suitable for IND and clinical phases
These factors ensure cost-effective bioanalytical services deliver long-term value, not short-term savings.
Reference
- Jemal, M. (2000). High-throughput quantitative bioanalysis by LC–MS/MS. Biomedical Chromatography, 14(6), 422–429.https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/1099-0801(200009)14:6%3C422::AID-BMC13%3E3.0.CO;2-Q
- Chambers, E., Wagrowski-Diehl, D. M., Lu, Z., & Mazzeo, J. R. (2007). Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. Journal of Chromatography B, 852(1–2), 22–34.https://www.sciencedirect.com/science/article/pii/S1570023207001173
- Shah, V. P., et al. (2000). Bioanalytical method validation—A revisit with a decade of progress. Pharmaceutical Research, 17(12), 1551–1557.
https://link.springer.com/article/10.1023/A:1007669411738 - European Commission. (2010). OECD Principles of Good Laboratory Practice (GLP).https://www.oecd.org/chemicalsafety/testing/oecd-series-on-principles-of-good-laboratory-practice-glp.htm
- Kelley, M., DeSilva, B., & Knight, J. (2019). Fit-for-purpose bioanalytical method development for early drug development. Bioanalysis, 11(7), 593–598.https://www.future-science.com/doi/10.4155/bio-2019-0034

