Introduction
When pharmaceutical and biotechnology companies seek Extractables and Leachables (E&L) testing services , one aspect is to have idea about the cost of Extractables and Leachables (E&L) testing. The cost of extractables and leachables testing is an depends on few major critical parameters such as Type of Container Closure system or delivery system, No. of components involved, Scope of E&L Testing, Analytical Techniques employed (GCMS, LCMS, ICPMS), Regulatory expectation and finally the method development criticality. In this article, we provide comprehensive information on all the above parameters, so that informed decision-making and cost-effective strategies can be achieved.
For organizations evaluating a vendor for E&L studies, ResolveMass Laboratories Inc. offers industry-leading analytical services that combine technical depth, regulatory compliance, and flexible pricing models. Learn more about our Extractables and Leachables Testing Services.
Summary
Understanding the cost of extractables and leachables testing is crucial for pharmaceutical, medical device, and biotechnology companies planning their product development budgets. The price typically ranges from $5,000 to $50,000+ per project, depending on multiple variables. Here’s what you need to know:
- Sample complexity directly impacts testing scope and analytical requirements
- Regulatory pathway (FDA, EMA, ISO standards) determines the depth of investigation needed
- Analytical methods employed (GC-MS, LC-MS, ICP-MS) significantly affect laboratory costs
- Study design (screening vs. controlled extraction studies) influences project duration and expenses
- Material contact surface area and extraction conditions multiply testing requirements
- Turnaround time expectations can add 20-50% to standard pricing for expedited services
- Follow-up toxicological assessments may double the initial analytical investment
1: What Are Extractables and Leachables?
Before diving into costs, it’s important to understand what these tests actually measure. Extractables are chemical compounds that can be extracted from materials under exaggerated conditions (aggressive solvents, elevated temperatures, extended contact times). Leachables are compounds that actually migrate into the drug product or medical device under normal storage and use conditions.
The distinction is critical: extractables studies identify potential risks, while leachables studies confirm what actually transfers to the patient.
Learn more about Extractables and Leachables through,https://resolvemass.ca/extractables-and-leachables-testing/
2: Why E&L Testing is Crucial in Pharmaceuticals
E&L testing ensures that any potentially harmful chemicals that migrate from packaging or manufacturing components into the drug product are identified and quantified. Regulatory agencies such as the FDA, EMA, and Health Canada require rigorous extractables and leachables testing to mitigate patient safety risks.
The cost of extractables and leachables testing can vary significantly depending on multiple parameters, which we explore below.
Also, if you want to learn in detail factors about various factors for Choosing the Best Lab for Extractables and Leachables (E&L) Testing in Montreal, Canada, or the United States.
3: Key Factors That Affect the Cost of Extractables and Leachables Testing
1. Type of Container or Delivery System
The container closure system or drug delivery device directly affects the cost of extractables and leachables testing. Complex systems like inhalers, pre-filled syringes, and transdermal patches require more extensive studies than simple glass vials. so first factor to be identified by the client is the identification of Container closure system.
2. Scope of Extractables vs. Leachables Studies
Extractables studies are typically conducted under exaggerated conditions, while leachables studies are done under real-time or accelerated stability conditions. Sometimes, the minor components observed below thresold values in Extractable study are leachables which needs to be identificed.
Explore our expertise in customized studies at ResolveMass E&L Testing Services.
3. Analytical Techniques Employed
Sometimes, it doesnot require to utilize all the instrument techniques, this can significantly reduce the overall E&L testing cost as the method development and instrument usage for that particular instrument would be reduced. Advanced techniques include:
- GC-MS: for volatile and semi-volatile organic compounds
- LC-MS/MS: for non-volatile compounds
- ICP-MS: for elemental impurities
- FTIR and UV-Vis: for material identification
To know more about these techniques, checkout our detailed article.
- Sometimes, microbilogical tests such as cytotoxicity, Hemocompatibility, Systemic Toxicity, Endotoxin Testing are also required as part of E&L Testing. This adds the cost of E&L testing.
Using multiple platforms for orthogonal confirmation increases both reliability and price.
4. Number of Components and Contact Materials for Cost of Extractables and Leachables Testing
More components in a system—stoppers, plungers, laminates, adhesives—mean more testing. This adds to sample prep time, extraction media, and analytical hours, clearly influencing the cost of extractables and leachables testing.
5. Regulatory Expectations and Submission Goals
Projects intended for regulatory submissions in the US, EU, or Canada often require customized protocols aligned with USP <1663> and <1664>, ISO 10993, and ICH Q3D. These require detailed documentation and QA oversight, increasing the cost of extractables and leachables testing.
We help clients navigate these frameworks at ResolveMass Laboratories.
6. Sample Throughput and Timelines for Cost of Extractables and Leachables Testing
Urgent timelines or parallel sample processing may require additional shifts, faster turnaround, or dedicated resources—all of which can increase the cost of extractables and leachables testing.
7. Method Development and Validation
New materials may need method development and validation, which is resource-intensive. For example, polymers with unknown extractables profiles may require LC-MS method development.
Get support with validated methods at ResolveMass’s E&L Testing Lab.
8. Reporting and Risk Assessment Support
Clients often require toxicological risk assessments, regulatory-ready reports, or justification packages. These scientific consulting services add value but can influence the cost of extractables and leachables testing.
If you want to learn more about Extractables and Leachables Testing in Canada: Best Practices for Risk Assessment
9. Quality Assurance and Data Integrity Protocols
21 CFR Part 11 compliance, electronic data transfer, and QA audit trails are crucial for GMP-compliant testing. Facilities with these capabilities offer reliable services—but the operational overhead may reflect in pricing.
Discover GMP testing infrastructure at ResolveMass Laboratories.
10. Batch Sizes and Number of Test Articles
Testing multiple batches or article replicates can add to extraction, analysis, and reporting workload—affecting the cost of extractables and leachables testing.
4: Primary Factors Influencing the Cost of Extractables and Leachables Testing
1. Sample Complexity and Material Composition
The complexity of your product-contact materials is the single largest driver of E&L testing costs. Simple single-polymer systems require far less investigation than multi-component assemblies with adhesives, coatings, gaskets, and multiple material layers.
Material complexity factors include:
- Number of different materials in contact with the product
- Presence of elastomers, adhesives, lubricants, or coatings
- Multi-layer or laminated structures
- Colored or printed components (inks add significant compound complexity)
- Previously characterized vs. novel materials
Cost impact: A simple polyethylene container might require $8,000-$15,000 for a complete extractables study, while a complex prefilled syringe system with multiple components could run $35,000-$60,000 or more.
2. Regulatory Requirements and Standards Compliance
Different regulatory pathways demand different levels of E&L investigation, directly impacting the cost of extractables and leachables testing. FDA guidance documents, USP chapters (particularly USP <1663>, <1664>, <665>, and <381>), ISO 10993 standards, and regional regulatory expectations all influence study design.
Regulatory considerations affecting cost:
| Regulatory Pathway | Testing Scope | Typical Cost Range |
|---|---|---|
| FDA Pharmaceutical (CDER) | Comprehensive E&L with safety qualification | $25,000 – $75,000+ |
| FDA Biologics (CBER) | Enhanced sensitivity, case-by-case approach | $30,000 – $80,000+ |
| Medical Device (ISO 10993-18) | Risk-based chemical characterization | $15,000 – $50,000+ |
| Generic Drug (ANDA) | Comparative E&L to reference product | $20,000 – $45,000+ |
| European Medicines Agency | Similar to FDA with regional variations | $25,000 – $70,000+ |
Cost impact: Regulatory-compliant studies following current FDA guidance typically cost 40-60% more than basic material characterization studies.
3. Analytical Methods and Instrumentation Required
The analytical techniques needed to detect, identify, and quantify extractables and leachables significantly influence testing costs. Modern E&L studies employ sophisticated instrumentation requiring specialized expertise and maintenance.
Common analytical methods and their applications:
- GC-MS (Gas Chromatography-Mass Spectrometry): Volatile and semi-volatile organic compounds
- LC-MS (Liquid Chromatography-Mass Spectrometry): Non-volatile, polar, and thermally labile compounds
- ICP-MS (Inductively Coupled Plasma-Mass Spectrometry): Elemental impurities and heavy metals
- HPLC-UV/DAD: Known compounds and quantitative analysis
- Headspace GC-MS: Volatile organic compounds in closed systems
- IC (Ion Chromatography): Ionic species and residual catalysts
Cost impact: Each analytical method adds $3,000-$12,000 to project costs. Comprehensive studies requiring 4-6 different techniques naturally cost more than targeted investigations using 1-2 methods.
4. Study Design: Screening vs. Controlled Extraction Studies
The type of extractables study you conduct directly affects the cost of extractables and leachables testing. Studies typically progress from initial screening to more rigorous controlled extraction studies.
Screening studies use aggressive solvents and conditions to provide a worst-case assessment of potential extractables. These are relatively cost-effective at $8,000-$20,000.
Controlled extraction studies use solvents and conditions that simulate actual drug product contact, providing more relevant data for safety assessments. These comprehensive studies typically cost $20,000-$45,000.
Leachables studies analyze the actual drug product or device extract over stability time points, requiring method development and validation. Costs range from $15,000-$40,000 depending on the number of time points and compounds monitored.
5. Extraction Conditions and Solvent Selection
The number of extraction conditions tested multiplies the analytical workload and cost. Regulatory guidance recommends testing with multiple solvents of varying polarity to ensure comprehensive extraction of diverse chemical compounds.
Typical extraction variables:
- Solvent polarity (aqueous, intermediate polarity, non-polar)
- Temperature (room temperature, 40°C, 60°C, reflux)
- Contact time (24 hours, 72 hours, extended)
- Surface area-to-volume ratios
- Static vs. dynamic extraction
Cost impact: Each additional extraction condition adds $2,000-$8,000 to the project cost, as all extracts must be analyzed using the full suite of analytical methods.
6. Surface Area and Sample Size Requirements
The ratio of material surface area to extraction solvent volume influences both the sensitivity of detection and the cost of extractables and leachables testing. Larger surface areas or multiple replicates require more materials, larger extraction vessels, and greater solvent volumes.
Cost impact: Projects requiring custom extraction vessels, large sample quantities, or special handling can add 15-30% to base analytical costs.
7. Compound Identification and Structure Elucidation
Detecting a compound is just the beginning—identifying unknown compounds drives significant additional costs. When analytical methods detect unidentified peaks, structural elucidation requires advanced techniques and expert interpretation.
Identification approaches:
- Library matching (least expensive)
- High-resolution mass spectrometry
- NMR (Nuclear Magnetic Resonance) spectroscopy
- Reference standard comparison
- Synthesis and confirmation
Cost impact: Each unknown compound requiring structural elucidation adds $1,500-$8,000 to project costs. Complex molecules or those lacking database matches require the most extensive (and expensive) investigation.
8. Toxicological Risk Assessment Requirements
Identifying compounds is only half the equation—you must also assess their safety. Toxicological risk assessments compare identified leachables to safety thresholds derived from the Permissible Daily Exposure (PDE), Threshold of Toxicological Concern (TTC), or existing toxicological data.
Safety assessment components:
- Literature searches for toxicological data
- PDE calculations based on available data
- Application of TTC principles for unknowns
- Safety qualification reports for regulatory submission
- Expert toxicologist review and recommendations
Cost impact: Basic toxicological assessments add $5,000-$15,000 to E&L studies. Comprehensive safety qualification packages for regulatory submissions can add $15,000-$40,000.
9. Turnaround Time and Expedited Services
Standard E&L studies typically require 8-16 weeks, but expedited timelines significantly increase the cost of extractables and leachables testing. Rush services require dedicated instrument time, overtime laboratory work, and priority scheduling.
Timeline options:
- Standard turnaround (12-16 weeks): Baseline pricing
- Expedited (6-8 weeks): 20-30% premium
- Rush (4-6 weeks): 40-50% premium
- Emergency (<4 weeks): 60-100% premium (if feasible)
Cost impact: Accelerated timelines for regulatory filing deadlines or product launch schedules can add $10,000-$30,000 to project costs.
10. Follow-Up and Confirmation Testing
Initial E&L studies often identify the need for additional investigation. Confirmation studies, method validation, stability testing of identified compounds, or additional extraction conditions all represent follow-up work that increases total costs.
Common follow-up needs:
- Method validation for quantitative leachables monitoring
- Stability-indicating leachables studies across shelf life
- Confirmatory testing with reference standards
- Additional material lots or suppliers
- Post-market surveillance testing
Cost impact: Follow-up testing can equal or exceed initial study costs, with method validation alone adding $15,000-$35,000.
5: Cost Optimization Strategies for Extractables and Leachables Testing
Strategic Planning Reduces Overall Investment
Early planning and phased approaches can significantly reduce the total cost of extractables and leachables testing while meeting regulatory requirements.
Cost-saving strategies:
- Start with screening studies to identify worst-case extractables before investing in comprehensive controlled extraction studies
- Leverage existing data on well-characterized materials to reduce testing scope
- Coordinate with material suppliers to obtain certificates of analysis and extractables data
- Phase testing to align with development milestones rather than conducting all studies upfront
- Use risk-based approaches to focus resources on highest-risk components
Selecting the Right Laboratory Partner
Choosing an experienced E&L testing laboratory with appropriate expertise and instrumentation ensures efficient studies and reduces the risk of costly study failures or regulatory questions.
What to look for:
- Direct experience with your product type and regulatory pathway
- In-house toxicology expertise for integrated risk assessment
- Modern, well-maintained analytical instrumentation
- Documented quality systems and regulatory inspection history
- Clear communication about study design, timelines, and costs
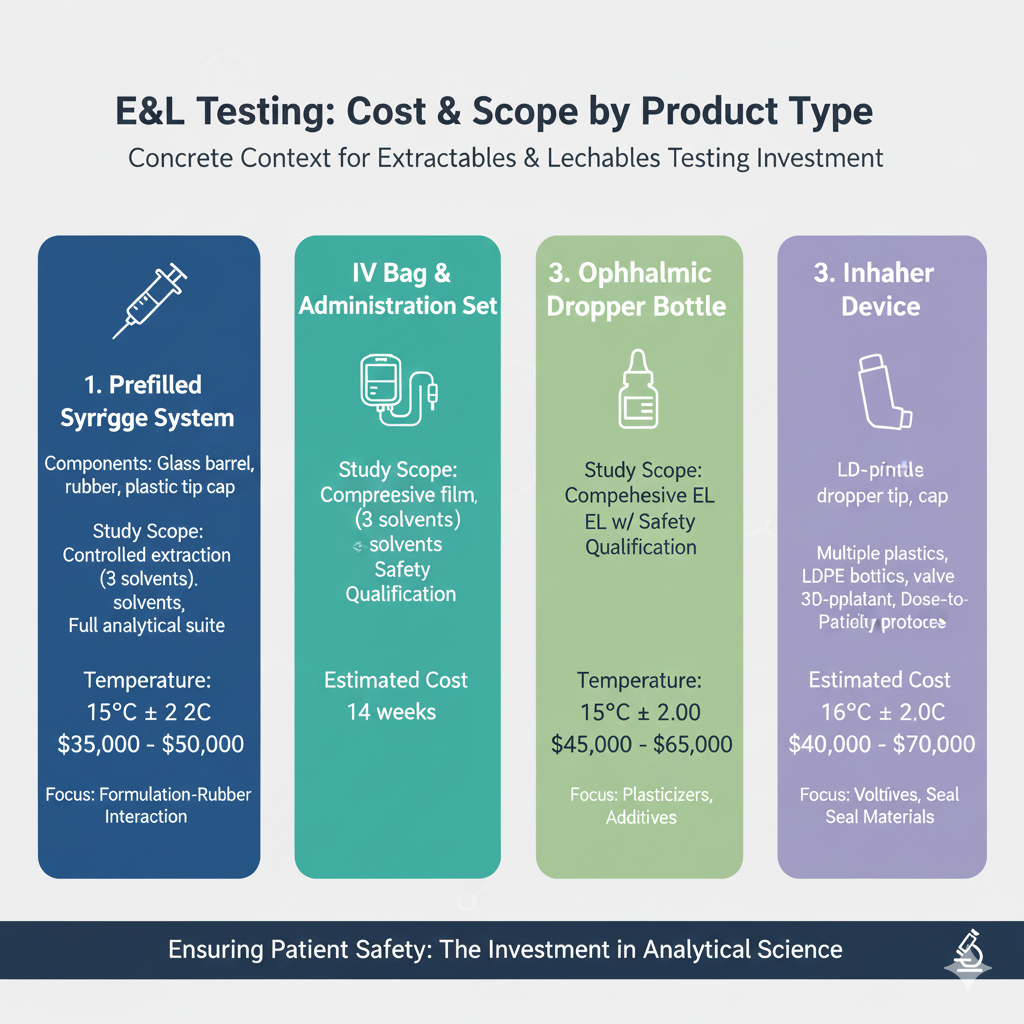
6: Real-World Cost Examples
To provide concrete context for the cost of extractables and leachables testing, here are representative examples from common product categories:
7: Understanding the Value Proposition
While the cost of extractables and leachables testing represents a significant investment, the value delivered extends far beyond regulatory compliance:
Risk mitigation: Identifying and addressing potential safety issues before market launch prevents costly recalls, regulatory actions, and patient harm.
Regulatory efficiency: Well-designed E&L studies conducted according to current guidance minimize regulatory questions and review cycles, accelerating approvals.
Competitive advantage: Comprehensive material characterization supports robust change control and supplier qualification, ensuring supply chain flexibility.
Patient safety: Ultimately, E&L testing ensures that your product meets the highest standards for patient safety and product quality.
8: How to Budget Smartly for E&L Testing
- Request tiered pricing for different testing levels
- Start with an extractables-only study to assess risk before proceeding to full leachables
- Use existing method libraries where possible
- Bundle testing services for cost savings across stability, E&L, and container testing
- Communicate intended use early to avoid unnecessary testing
Learn how ResolveMass helps clients build cost-effective E&L testing plans: Explore Services.
9: Why Choose ResolveMass for E&L Testing
ResolveMass Laboratories Inc. brings:
- A proven track record in extractables and leachables testing for complex devices
- Access to cutting-edge instrumentation including LC-MS, GC-MS, and ICP-MS
- Compliance with USP <1663>, <1664>, and ISO 10993-18
- Responsive project management and detailed regulatory reporting
We’ve supported preclinical to commercial-stage programs with tailored E&L strategies that deliver quality insights and cost control.
Let us design your next E&L study: Request a Quote
Also Read
Conclusion
Understanding the cost of extractables and leachables testing is essential for pharmaceutical packaging and device developers. From component complexity to analytical techniques and reporting expectations, many factors shape the final price. Partnering with a knowledgeable lab like ResolveMass Laboratories ensures that your testing strategy is not only scientifically robust but also financially optimized.
Learn more about our E&L Testing Services or get in touch to request a quote today.
Contact Us:
ResolveMass Laboratories Inc.: Comprehensive Scientific Expertise You Can Rely On
ResolveMass Laboratories Inc. is a trusted Canadian contract research organization offering a wide spectrum of specialized services spanning polymer synthesis, advanced analytical testing, and custom organic synthesis. With over a decade of experience supporting pharmaceutical, biotech, and industrial clients, we bring scientific precision and regulatory insight to every project. Our core capabilities include Polymer Synthesis and Characterization, Peptide Characterization, Organic Synthesis, Nitrosamine Testing and Analysis, PFAS Testing, and Extractable & Leachable Studies, as well as a broad suite of analytical techniques such as HPLC, GC-MS, MALDI-TOF, NMR, and FTIR.
Our multidisciplinary team includes chemists, analytical scientists, and regulatory experts with advanced academic and industry backgrounds. We excel at developing customized, high-quality solutions—whether you need innovative polymer designs, impurity profiling, or confirmatory testing that meets global regulatory standards.
Clients across North America choose ResolveMass Laboratories for our deep technical knowledge, commitment to quality, and ability to deliver reproducible, reliable data that drives confident decision-making. When precision, innovation, and trust matter—ResolveMass is your partner of choice.

