✅ Summary: Drug Substance vs Drug Product CMC – IND and NDA Perspectives
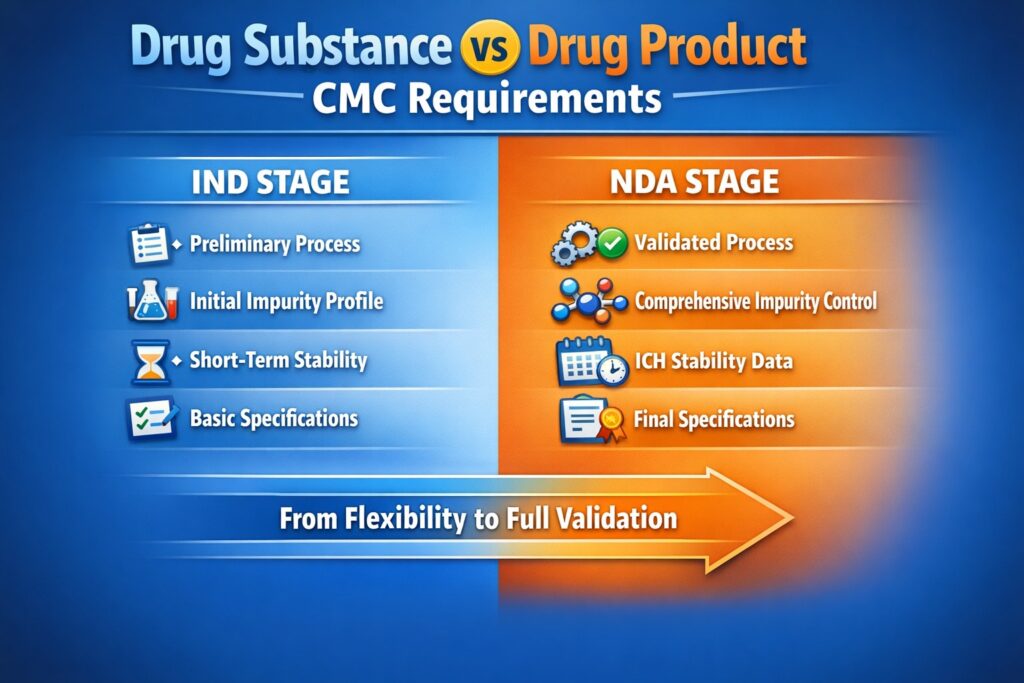
- CMC for Drug Substance and Drug Product differ significantly in IND vs NDA stages.
- Drug Substance CMC focuses on synthesis, control strategy, and stability; Drug Product CMC covers formulation, container-closure, and performance.
- IND requires minimal but justifiable data; NDA requires comprehensive, validated datasets.
- Regulatory expectations escalate across the development timeline.
- Global harmonization (ICH Q7/Q8/Q11) drives strategic integration of CMC across programs.
- Lifecycle management, bridging strategies, and risk mitigation are critical for CMC success.
- Focus keyword “Drug substance Chemistry, Manufacturing, and Controls” used throughout as per SEO guidelines.
🔍 Introduction
A comprehensive understanding of Drug substance Chemistry, Manufacturing, and Controls is key to success at every regulatory milestone, particularly during IND and NDA submissions. Regulatory agencies set specific expectations at each stage, which must be addressed with increasing detail as development advances.
This guide compares CMC requirements for Drug Substance and Drug Product, explaining how priorities shift from early-stage feasibility to late-stage control and validation. These insights are essential for maintaining compliance and accelerating development.
Explore our comprehensive services: Chemistry, Manufacturing, and Controls (CMC) Services
🧬 Drug Substance CMC Requirements in IND and NDA
🔹 IND Stage – Drug Substance CMC Requirements
In the IND phase, the focus of Drug Substance CMC is on safety and scientific justification. Regulators accept early-stage flexibility, provided that patient safety is ensured.
The manufacturing process doesn’t need to be fully validated at this stage. However, the synthetic route must be described clearly, and materials should meet initial quality standards. Basic analytical data and short-term stability support the safe use of the product in clinical trials.
Learn more about early-stage requirements: IND CMC Services
Key Deliverables:
- Description of synthetic route and manufacturing process (non-validated)
- Preliminary impurity profile
- Batch data from toxicology and clinical trial lots
- Initial specifications with acceptance criteria
- Short-term stability data
Providing details on raw materials and early risk assessments helps strengthen the submission and prepares for future stages.
🔹 NDA Stage – Drug Substance CMC Requirements
At the NDA stage, Drug substance Chemistry, Manufacturing, and Controls must be fully developed. Regulatory agencies expect complete validation, impurity control, and documented process consistency.
Processes must be scalable, reproducible, and thoroughly understood. Impurity profiles should include fate and purge studies. Stability testing under ICH conditions is required to define shelf life, and analytical methods must be fully validated.
Compare the transitions: IND vs NDA CMC Requirements
Key Deliverables:
- Fully validated and scalable manufacturing process
- Impurity control strategy with fate and purge studies
- Final specifications per ICH Q6A/B
- Long-term ICH-compliant stability data
- Validated analytical methods
- Justified regulatory starting materials (RSM)
This stage demands a comprehensive, risk-based control strategy backed by strong scientific rationale.
✅ Table: Drug Substance CMC Requirements (IND vs NDA)
| Component | IND Stage | NDA Stage |
|---|---|---|
| Process Description | Preliminary | Validated and scalable |
| Impurity Profile | Initial assessment | Detailed with fate and purge studies |
| Stability | Short-term, indicative | Long-term under ICH conditions |
| Specifications | Tentative | Final and ICH-compliant |
| Control Strategy | Emerging | Fully developed and justified |
| Analytical Methods | Fit-for-purpose | Fully validated |
💊 Drug Product Chemistry, Manufacturing, and Controls (CMC): IND and NDA
🔹 IND Stage – Drug Product CMC Requirements
In the IND phase, the priority for Drug Product CMC is to ensure the formulation is appropriate for clinical testing and does not pose safety risks. Flexibility is allowed if the formulation is justified and safe for use.
The product may be a prototype, but its ingredients and function must be well described. Basic container-closure compatibility and stability for the clinical trial period must be documented.
Key Deliverables:
- Description of clinical trial formulation
- Container-closure compatibility data
- Short-term stability under storage conditions
- Preliminary manufacturing process description
Supporting information, like excipient rationale and in-process controls, can enhance the regulatory review process.
Strategic planning for your submission: IND CMC Strategy
🔹 NDA Stage – Drug Product CMC Requirements
At this stage, the product must be final and ready for commercial production. The formulation should be scientifically justified, and manufacturing must be validated with defined Critical Quality Attributes (CQAs) and Process Parameters (CPPs).
Complete dissolution testing and extractables/leachables data for packaging are required. Specifications must be final, justified, and based on clinical relevance.
Understand the final submission criteria: NDA CMC Requirements
Key Deliverables:
- Finalized formulation with justification
- Validated manufacturing process with defined CPPs and CQAs
- In vitro performance data (dissolution and bioavailability)
- Comprehensive container-closure studies
- Long-term ICH-compliant stability data
- Final, clinically relevant specifications
✅ Table: Drug Product CMC Requirements (IND vs NDA)
| Component | IND Stage | NDA Stage |
|---|---|---|
| Formulation | Prototype | Finalized with justification |
| Process Validation | Not required | Mandatory |
| Stability | Short-term | Long-term, ICH-compliant |
| Performance Testing | Minimal | Dissolution and release profiling |
| Container Closure Compatibility | Basic compatibility | Full extractables and leachables |
| Specifications | Interim | Final, clinically relevant |
📈 Regulatory Landscape for CMC: IND to NDA
As development progresses, regulators expect more detailed and validated CMC data. While IND focuses on safety and early feasibility, NDA ensures consistent manufacturing and long-term product quality.
Optimize your regulatory path: CMC Services for IND and NDA
IND CMC Focus:
- Ensuring patient safety
- Supporting early clinical studies
- Scientific justification
NDA CMC Focus:
- Demonstrating quality and control
- Validated processes and specifications
- Lifecycle and risk management
🔄 Bridging and Lifecycle Management Strategies
Bridging between IND and NDA is crucial, especially when changes in formulation, process, or manufacturing site occur. A clear strategy helps avoid delays and supports smooth regulatory review.
Comparative data and bridging stability studies demonstrate consistency across development stages. Risk assessments and updated control strategies show regulators that potential issues are proactively managed.
Avoid common pitfalls: CMC Deficiencies in IND & NDA
🌐 Harmonized Global Expectations in Drug Substance Chemistry, Manufacturing, and Controls
International regulatory agencies (e.g., FDA, EMA, PMDA) promote harmonization through ICH guidelines like Q7, Q8, and Q11. This global approach supports consistent expectations and reduces duplication.
Following harmonized guidelines allows for efficient development, easier global submissions, and faster regulatory approvals, improving overall drug lifecycle planning.
🔬 Risk-Based Approaches in Drug Substance Chemistry, Manufacturing, and Controls
While not required during IND, risk-based and Quality by Design (QbD) principles are important at the NDA stage. These demonstrate control, understanding, and proactive quality assurance.
Key elements include identifying CQAs, defining design space, using Process Analytical Technology (PAT), and developing robust control strategies. These tools support long-term consistency and regulatory trust.
📌 CMC Success Tips for IND and NDA
- Start impurity identification and control strategies early
- Justify starting materials with clear scientific reasoning
- Align Drug Product specs with clinical safety and PK data
- Keep documentation consistent and traceable across phases
- Use collected stability data to extend shelf life where applicable
Ensure testing accuracy: Analytical Method Development for IND and NDA
🔚 Conclusion
Strong planning and execution of Drug substance Chemistry, Manufacturing, and Controls is essential from the IND through NDA stages. As requirements evolve, so must your strategies for managing both Drug Substance and Drug Product.
By following global regulatory standards, applying risk-based development, and maintaining lifecycle control, your CMC package can support smooth approvals. At every step, ResolveMass Laboratories offers expert support to navigate these complex requirements with confidence.
🔗 Need Expert CMC Support?
Partner with ResolveMass Laboratories for IND/NDA CMC services tailored to your molecule.
📩 Contact us now
FAQs – Drug Substance vs Drug Product CMC in IND and NDA
The main difference lies in the level of detail and validation required. During the IND stage, the focus is on demonstrating feasibility and ensuring patient safety with preliminary data. By the NDA stage, regulatory agencies require full validation, comprehensive control strategies, and consistent manufacturing processes.
No, process validation is not a requirement at the IND stage. At this early phase, regulators accept non-validated processes as long as they are scientifically sound and safe for clinical use. Full process validation is expected during NDA to confirm consistent performance at commercial scale.
Yes, it is common for formulations to evolve between IND and NDA phases. However, any changes must be well-justified through bridging studies, comparability assessments, and stability data to ensure that the final product maintains safety, quality, and efficacy.
For IND submissions, short-term stability data is generally sufficient to support the proposed duration of clinical use. This data should show that the drug remains stable under recommended storage conditions for the trial period, ensuring patient safety during early studies.
Quality by Design (QbD) becomes increasingly important at the NDA stage. It helps demonstrate thorough process understanding, identification of critical parameters, and proactive risk management, all of which contribute to building regulatory confidence in the product’s long-term reliability.
Reference
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas


