
Introduction:
Extractables and Leachables (E&L) Testing for Inhalation and Nasal Drug Products has become a regulatory imperative in 2025. Given the direct contact of these drug products with respiratory pathways, any leachable compound from container closure systems, delivery devices, or packaging materials can significantly impact patient safety. E&L testing for inhalation and nasal drug products ensures that no toxic chemical migrates into the formulation. ResolveMass Laboratories Inc., with advanced analytical capabilities and compliance-driven strategies, is trusted by leading pharmaceutical and biotech companies for comprehensive E&L testing services.
Check out our full E&L testing services here – Choosing the Right E&L Testing Service Provider
Summary
Key Takeaways:
- E&L testing is mandatory for inhalation and nasal drug products to meet FDA and EMA requirements
- Extractables studies identify potential leachables under controlled laboratory conditions
- Leachables testing evaluates actual migration under real-world storage conditions
- Testing protocols include chemical characterization, toxicological assessment, and risk evaluation
- Specialized analytical techniques (GC-MS, LC-MS, ICP-MS) are essential for accurate detection
- Proper study design prevents costly regulatory delays and ensures patient safety
- Expert laboratories provide comprehensive testing from method development to regulatory submission
1: Understanding the Regulatory Landscape
E&L Testing for Inhalation and Nasal Drug Products and Global Guidelines
E&L Testing for Inhalation and Nasal Drug Products must comply with stringent regulations including:
- USP <1663> and <1664>: For extractables and leachables assessment
- FDA Guidance for Industry on OINDP (Orally Inhaled and Nasal Drug Products)
- ICH Q3D Elemental Impurities
- ISO 10993-18: For medical device compatibility and safety
These guidelines mandate risk assessment, controlled extraction studies, and toxicological evaluation of identified leachables.
Want to learn more about regulatory compliance in E&L testing?
2: Step-by-Step Process of E&L Testing for Inhalation and Nasal Drug Products
Step 1: Material Selection and Risk Assessment
ResolveMass begins every E&L Testing for Inhalation and Nasal Drug Products project with a thorough evaluation of materials used in components such as:
- Metered Dose Inhalers (MDIs)
- Dry Powder Inhalers (DPIs)
- Nasal Sprays
- Valves, canisters, gaskets, actuators
Each material undergoes risk classification to determine its likelihood of releasing extractables and leachables under expected conditions.
Need a study tailored to your product? Start here – Customized E&L Testing Solutions for Your Industry
Step 2: Controlled Extraction Studies (USP <1663>)
Controlled Extraction is the cornerstone of extractables profiling. ResolveMass uses aggressive solvents, varied temperatures, and time durations to force the extraction of potential chemicals. Common solvents include:
- Hexane
- Isopropanol
- Water
- 50% ethanol
Step 3: Analytical Testing (LC-MS, GC-MS, ICP-MS)
ResolveMass deploys the following advanced analytical tools:
LC-MS (Liquid Chromatography–Mass Spectrometry)
- For semi-volatile polar extractables such as plasticizers and degradation products
GC-MS (Gas Chromatography–Mass Spectrometry)
- Ideal for volatile organic compounds including residual solvents and aromatic hydrocarbons
ICP-MS (Inductively Coupled Plasma Mass Spectrometry)
- Detects elemental impurities like lead, cadmium, arsenic, and mercury
These instruments are crucial for achieving method sensitivity in E&L Testing for inhalation and nasal drug products. Don’t miss the detailed insights on when to use techniques like LC-MS, GC-MS, and ICP-MS—and how they impact your study results.
👉 Explore the full article on E&L analytical techniquesStep 4: Leachables Study Under Simulated Use Conditions (USP <1664>)
Using real-time or accelerated stability conditions, ResolveMass evaluates actual migration of extractables into drug formulation. These leachables studies are aligned with shelf-life duration and temperature/humidity conditions.
Samples are analyzed at multiple time points: T=0, 3 months, 6 months, and up to 24 months.
Step 5: Toxicological Risk Assessment and Reporting
Every compound identified during E&L Testing for Inhalation and Nasal Drug Products undergoes toxicological evaluation. ResolveMass leverages qualified toxicologists to assess:
- PDE (Permitted Daily Exposure)
- TTC (Threshold of Toxicological Concern)
- Genotoxicity and carcinogenicity
Comprehensive reports with ICH and FDA-ready formatting are provided to clients.

See how we solved a real-world leachables challenge –
See our recent case study – Case Study: Enhancing Product Safety Through Comprehensive E&L Testing
3: Why E&L Testing for Inhalation and Nasal Drug Products Is Critical
E&L testing for inhalation and nasal drug products is essential because these formulations deliver medication directly to the respiratory system, where even trace amounts of toxic compounds can cause immediate and serious adverse effects. The lungs have extensive surface area, rich blood supply, and high absorption potential, making them particularly vulnerable to chemical contaminants.
Unique Risk Factors for Inhalation Products
Direct Respiratory Exposure: Unlike oral medications that undergo first-pass metabolism, inhaled drugs—and any leachables present—bypass digestive and hepatic processing, entering systemic circulation directly through lung tissue.
Device Complexity: Inhalation devices contain multiple materials (elastomers, plastics, metals, lubricants, adhesives) that contact the formulation, increasing the potential for chemical migration.
Patient Populations: Many inhalation products treat chronic conditions in vulnerable populations including children, elderly patients, and individuals with compromised respiratory function.
Regulatory Requirements
- FDA Guidance: The FDA’s Container Closure Systems guidance and product-specific guidelines require comprehensive E&L assessment
- EMA Requirements: European Medicines Agency mandates E&L studies per ICH Q3C and Q3D guidelines
- ISO Standards: ISO 10993 series provides biocompatibility testing frameworks
- USP Guidelines: USP <1663> and <1664> chapters offer detailed E&L testing methodologies
4: Why Choose ResolveMass Laboratories Inc. for E&L Testing for Inhalation and Nasal Drug Products?
Deep Experience with OINDP Studies
ResolveMass Laboratories has performed over 200 E&L studies, many involving inhalation and nasal delivery platforms. Our expertise covers:
- pMDI and DPI devices
- Nasal sprays with biologics
- Combination products
Rapid Turnaround with Regulatory-Grade Documentation
We understand IND/NDA submission timelines and provide:
- Draft reports within 2-3 weeks
- Final ICH and FDA-submission-ready documentation
- On-call scientific liaisons for regulatory meetings
End-to-End Capabilities Under One Roof
From material characterization to LC-MS/GC-MS/ICP-MS testing and toxicology reporting — all services are performed at ResolveMass’ state-of-the-art facility in Canada.
5: Use Cases: When Is E&L Testing for Inhalation and Nasal Drug Products Mandatory?
- New Product Launch: IND filing for a new nasal or inhaled formulation
- Device Change: Switching plastic components in an MDI or DPI
- Material Change: Supplier switch or resin change
- Regulatory Response: Addressing FDA/EMA E&L queries during review
Learn more about leachables in medical device components – Case Study: Identifying and Mitigating Leachables in Medical Devices
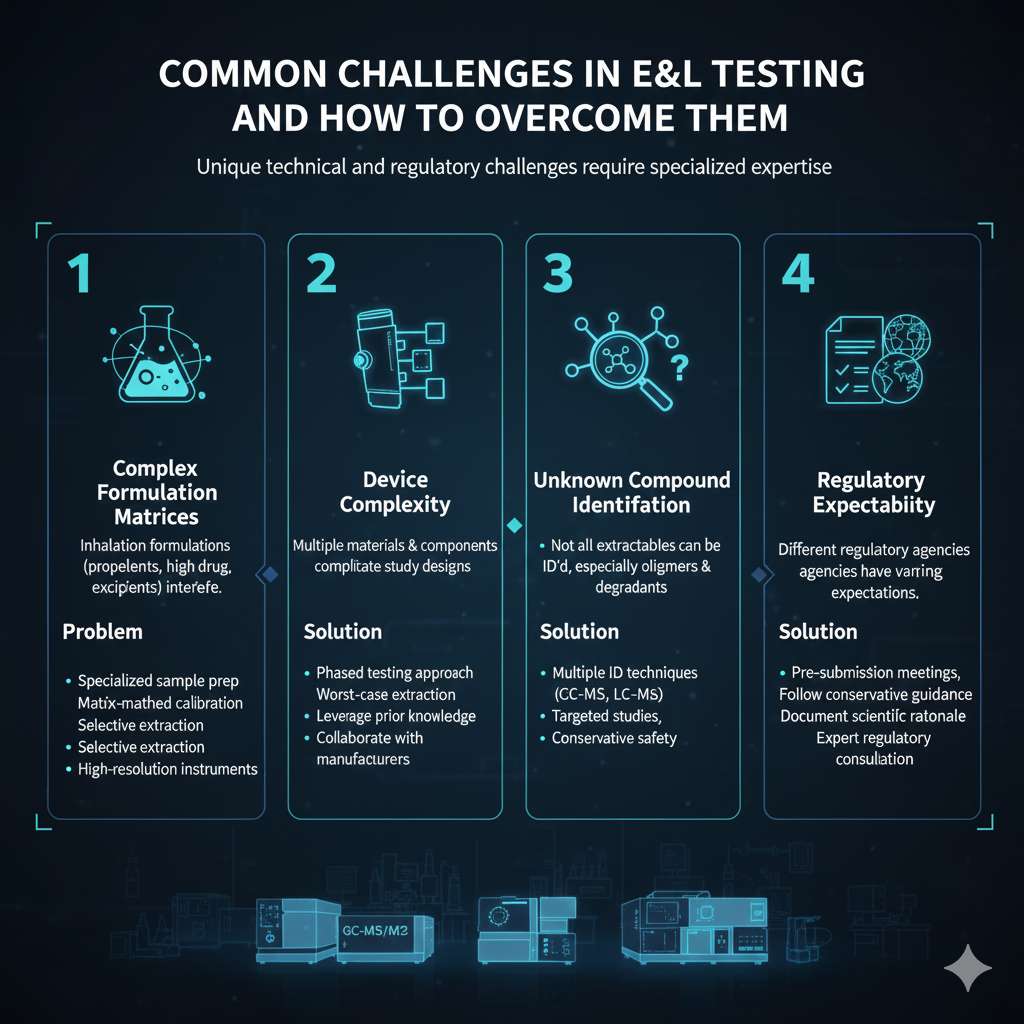
6: Common Challenges in E&L Testing and How to Overcome Them
E&L testing for inhalation and nasal drug products presents unique technical and regulatory challenges that require experienced problem-solving and specialized expertise.
Challenge 1: Complex Formulation Matrices
Problem: Inhalation formulations (propellants, high drug concentrations, excipients) interfere with analytical detection of low-level leachables.
Solution:
- Develop specialized sample preparation techniques
- Use matrix-matched calibration standards
- Employ selective extraction and cleanup procedures
- Utilize high-resolution analytical instruments
Challenge 2: Device Complexity
Problem: Multiple materials and components create complicated extraction study designs.
Solution:
- Conduct phased testing approach (components then assembled device)
- Use worst-case extraction conditions
- Leverage prior knowledge from similar devices
- Collaborate with device manufacturers
Challenge 3: Unknown Compound Identification
Problem: Not all extractables can be definitively identified, especially oligomers and degradants.
Solution:
- Use multiple identification techniques (GC-MS, LC-MS, NMR)
- Conduct targeted studies on suspected compounds
- Apply structure-activity relationship (SAR) analysis
- Use conservative safety assessments for unidentified compounds
Challenge 4: Regulatory Expectation Variability
Problem: Different regulatory agencies may have varying expectations.
Solution:
- Engage in pre-submission meetings
- Follow most conservative guidance when in doubt
- Document scientific rationale clearly
- Seek expert regulatory consultation
Frequently Asked Questions
Q1: How long does an E&L Testing for Inhalation and Nasal Drug Products project take? Typically, extractables studies take 3-4 weeks. Leachables studies may range from 3 to 24 months based on storage condition simulation.
Q2: Can ResolveMass support GLP and cGMP testing? Yes, all E&L Testing for Inhalation and Nasal Drug Products is performed under GLP/cGMP standards with full traceability and audit readiness.
Q3: What is the cost of a typical E&L study? Costs depend on the number of components, number of solvents used, and toxicological review. Contact ResolveMass for custom quotes.
Please visit our article for more information about cost of E&L testing in 2025.
Conclusion:
E&L Testing for Inhalation and Nasal Drug Products is not just a regulatory checkbox—it is a critical tool to ensure patient safety and compliance. By partnering with ResolveMass Laboratories Inc., pharmaceutical developers gain access to analytical rigor, regulatory insight, and trusted technical expertise. We help you de-risk your development program with confidence.
Learn more about our E&L Testing Capabilities
Contact ResolveMass Laboratories
ResolveMass Laboratories Inc.: Comprehensive Scientific Expertise You Can Rely On
ResolveMass Laboratories Inc. is a trusted Canadian contract research organization offering a wide spectrum of specialized services spanning polymer synthesis, advanced analytical testing, and custom organic synthesis. With over a decade of experience supporting pharmaceutical, biotech, and industrial clients, we bring scientific precision and regulatory insight to every project. Our core capabilities include Polymer Synthesis and Characterization, Peptide Characterization, Organic Synthesis, Nitrosamine Testing and Analysis, PFAS Testing, and Extractable & Leachable Studies, as well as a broad suite of analytical techniques such as HPLC, GC-MS, MALDI-TOF, NMR, and FTIR.
Our multidisciplinary team includes chemists, analytical scientists, and regulatory experts with advanced academic and industry backgrounds. We excel at developing customized, high-quality solutions—whether you need innovative polymer designs, impurity profiling, or confirmatory testing that meets global regulatory standards.
Clients across North America choose ResolveMass Laboratories for our deep technical knowledge, commitment to quality, and ability to deliver reproducible, reliable data that drives confident decision-making. When precision, innovation, and trust matter—ResolveMass is your partner of choice.
References:

