Introduction: The Importance of Forced Degradation in Understanding Alectinib Stability
Forced degradation plays a central role in the development and quality assurance of modern drug substances, and this is especially true for chemically intricate oncology agents like Alectinib Hydrochloride. By deliberately subjecting the molecule to controlled stress conditions, researchers can uncover how Alectinib responds to environmental and chemical challenges that may arise during manufacturing, distribution, and long-term storage. These stress studies reveal its inherent degradation pathways, expose previously unrecognized impurities, and verify that analytical procedures are capable of detecting all relevant degradants throughout the product’s lifecycle. Regulatory bodies such as the FDA and EMA require comprehensive stress-testing data to comply with ICH Q1A(R2), Q3A(R2), and Q3B(R2) guidelines, making degradation profiling an indispensable part of the development process.
For a complex kinase inhibitor like Alectinib Hydrochloride—used in the treatment of ALK-positive non–small cell lung cancer (NSCLC) and known for its effectiveness in patients showing resistance to earlier-generation ALK inhibitors—understanding its stability behavior is critical. The molecule’s multi-ring structure, heteroatomic functional groups, and susceptibility to oxidative transformation demand detailed investigation to map potential degradation routes. Given its pivotal role in targeted cancer therapy, establishing a complete impurity profile is necessary to ensure consistent clinical performance and minimize any risks posed by structurally related degradation products.
Comprehensive impurity knowledge also informs formulation teams during excipient selection and packaging design. Because Alectinib contains functional groups sensitive to oxidation and light, assessing its degradation under various conditions helps manufacturers implement processing controls that preserve the drug’s structural integrity. This insight is essential for designing a stability-indicating method and for predicting challenges that may become more pronounced during scale-up or commercial production.
This case study presents an integrated forced degradation and impurity characterization workflow specifically applied to Alectinib Hydrochloride. Through the combined use of LC–MS, NMR, FTIR, and preparative HPLC, four previously unreported oxidative impurities were successfully isolated and structurally identified. The approach reflects the comprehensive analytical capabilities provided by ResolveMass Laboratories, encompassing forced degradation studies, impurity isolation, and full structural elucidation.
The study demonstrates how advanced and complementary analytical techniques can uncover subtle degradation processes that may go unnoticed in routine testing. It also emphasizes the value of specialized instrumentation early in the development timeline, where a deep understanding of impurity behavior can significantly influence formulation strategy, process design, and long-term product stability.
Experimental Overview of Alectinib Hydrochloride
Forced Degradation Conditions Evaluated
Following ICH Q1A(R2) and Q1B guidelines, Alectinib Hydrochloride underwent multiple stress conditions to evaluate its stability profile:
- Acidic hydrolysis: 5 M HCl at 60 °C for 30 min
- Basic hydrolysis: 5 M NaOH at 60 °C for 30 min
- Oxidative stress: 30% H₂O₂ at 60 °C for 30 min
- Photolytic degradation: UV & visible light exposure for 4 hours (solution & solid-state)
- Thermal stress: 80 °C for 2 hours
Interestingly, no significant degradation occurred under acidic, basic, photolytic, or thermal conditions. Only oxidative conditions generated notable degradants, revealing a specific vulnerability of the molecule. This observation highlights the importance of targeted stress studies rather than assuming all conditions will produce similar outcomes.
Such results also help stability teams prioritize control strategies that focus on oxygen-related degradation. The information becomes critical when selecting antioxidants, packaging, and storage recommendations that prevent oxidative stress.
Analytical Techniques Used in Alectinib Hydrochloride
To fully detect, separate, and characterize the oxidative degradation products, several complementary analytical technologies were employed:
- HPLC / LC–MS/MS (Waters Alliance 2695 & Micromass ZQ):
Enabled initial impurity detection and provided mass fragmentation data to predict structural changes. - Prep-HPLC (Shimadzu LC-20AP):
Allowed isolation of individual impurities using a C18 250 × 25 mm, 5 µm column, ensuring adequate purity for NMR and FTIR. - NMR (Bruker AVANCE 400 MHz):
¹H, ¹³C-BBD, DEPT-90/135 confirmed structural modifications and supported complete elucidation of degradants. - FTIR Spectroscopy:
Provided functional-group confirmation, particularly for CN stretch, carbonyls, and amide bands.
These techniques work synergistically, ensuring no degradation product goes unidentified. The combination of chromatographic separation and spectroscopic interpretation is essential when dealing with closely related impurities.
This multi-layered approach helps guarantee regulatory-ready data and supports accurate impurity reporting for submissions.
Detection & Isolation Workflow of Alectinib Hydrochloride
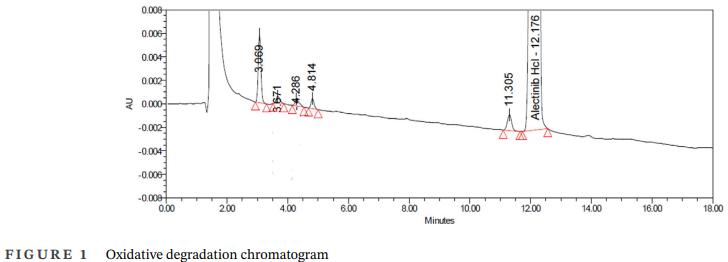
Oxidative samples produced four distinct impurity peaks in the chromatogram (Figure 1). Each peak showed unique retention behavior, suggesting they represented structurally different species. They were collected using preparative HPLC under optimized chromatographic conditions.
The purified fractions were subsequently analyzed using LC–MS, NMR, and FTIR to determine their identity and degradation mechanisms. This structured workflow ensures that every impurity is studied thoroughly before concluding its relevance or potential toxicity.
Such isolation-to-characterization pipelines are now considered best practice for complex small-molecule APIs.
For more insights into implementing similar workflows, explore:
https://resolvemass.ca/hplc-analysis-2/
https://resolvemass.ca/analytical-method-development/
https://resolvemass.ca/forced-degradation-testing-service-in-pharmaceuticals/
Identification and Structural Elucidation of Novel Impurities in Alectinib Hydrochloride
The oxidative stress study produced four novel degradation products. Three of these displayed similar molecular mass (m/z ~499) but had different retention times, confirming they were constitutional isomers rather than identical structures. Their detailed characterization is described below, based entirely on experimental interpretation.
Understanding these subtle differences is crucial because isomers often vary significantly in reactivity and potential biological impact. The data also reinforces why high-resolution analytical tools are indispensable for oncology drug development.
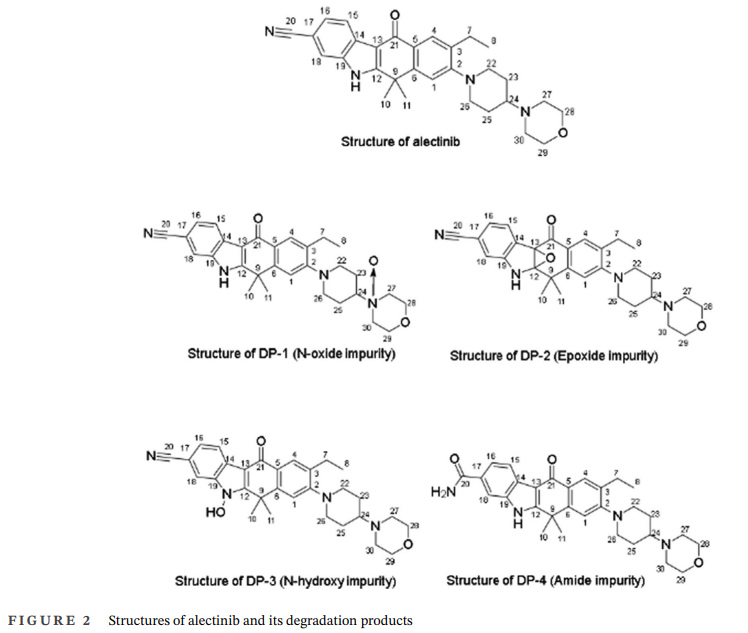
DP-1: N-Oxide Impurity
LC–MS Findings:
- Molecular ion at m/z 499.15
- Daughter ion at m/z 396.26, indicating loss of the morpholine ring + O atom
APCI Results:
- Peaks at m/z 499.12 and 483.22, reflecting typical N-oxide deoxygenation behavior
NMR Observations:
- Proton shifts between 2.14–4.25 ppm consistent with N-oxide formation in the piperidine–morpholine region
FTIR:
- CN stretch remained at ~2217 cm⁻¹
Conclusion:
Oxygen insertion occurred at the nitrogen of the morpholine moiety.
The formation of N-oxides is common in oxidative pathways, especially in tertiary amine-containing drugs. Identifying this impurity helps determine whether antioxidants or oxygen-limiting conditions are necessary during processing.
Such findings also help predict long-term degradation trends under accelerated stability conditions.
DP-2: Epoxide Impurity
LC–MS:
- Molecular ion at m/z 499.26
- Fragment at m/z 412.28, consistent with loss of morpholine (86 Da)
NMR:
- Merging of methyl proton signals (1.0–2.0 ppm) suggesting altered shielding at C-5/C-6
Mechanistic Insight:
Likely epoxidation between C-12 and C-13 of the carbazole core.
Conclusion:
An epoxide ring formed within the benzo[b]carbazole system.
Epoxide formation is significant because such intermediates may be reactive or unstable under certain conditions. Recognizing this pathway enables manufacturers to refine stress controls during transport and storage.
It also highlights regions of the molecule that may require protective formulation strategies.
DP-3: N-Hydroxy Impurity
LC–MS:
- Fragment at m/z 481.99, confirming loss of OH
MS/MS:
- Fragment at m/z 395.25, indicating loss of OH and morpholine
NMR:
- Additional deshielding near positions 12–19, consistent with N-OH functionalization
Conclusion:
The impurity represents hydroxylation of a key nitrogen atom in the aromatic ring system.
N-hydroxy impurities often arise during strong oxidative conditions and may have unique reactivity. Detecting this impurity helps track oxygen-driven transformation routes and assess whether formulation adjustments are required.
Its presence also reinforces the need for precise control over oxidizing agents during synthesis.
DP-4: Amide Impurity
LC–MS:
- Molecular ion at m/z 501.27, slightly higher than the other DPs
NMR:
- Characteristic –CONH₂ proton signal at ~7.3 ppm
FTIR:
- CN band (~2219 cm⁻¹) disappeared
- Strong amide carbonyl signal appeared
Conclusion:
The nitrile group underwent oxidative conversion to a primary amide.
Nitrile-to-amide conversion is a known oxidative pathway and may influence long-term impurity profiles. Understanding this route helps establish impurity acceptance criteria and storage recommendations.
This insight also supports proactive quality control during scale-up.
Regulatory and Scientific Relevance
Forced degradation studies and impurity identification are critical for meeting global regulatory expectations.
Alignment with ICH Guidelines
- ICH Q1A(R2): Outlines stress-testing requirements
- ICH Q1B: Addresses photostability testing
- ICH Q3A/Q3B: Requires identification and qualification of impurities above defined thresholds
Alectinib’s oxidative sensitivity underscores the need for analytical methods that can detect minor degradants, distinguish structural isomers, and characterize novel impurities before regulatory submission. Robust data supports safe impurity specifications and ensures consistent product quality.
These results also offer valuable guidance for formulation development and packaging design, especially when oxygen exposure poses a risk.
More resources on regulatory-aligned method development:
https://resolvemass.ca/method-development-vs-method-validation/
https://resolvemass.ca/what-is-analytical-method-development/
https://resolvemass.ca/forced-degradation-testing-in-pharma/
Key Learnings and ResolveMass Expertise
This case study highlights the importance of integrated analytical platforms when evaluating complex degradation patterns:
- LC–MS/MS for sensitive impurity detection
- Preparative HPLC for isolating closely related compounds
- NMR for complete structural elucidation
- FTIR for functional-group verification
At ResolveMass Laboratories, this workflow forms the backbone of our impurity profiling services. Our team supports:
- Isolation of unknown impurities
- Impurity profiling for Alectinib and related TKIs
- Forced degradation study execution in Canada and the U.S.
- Preparation of regulatory-ready documentation
- Stability-indicating method development and validation
Our capabilities help drug developers address impurity challenges early in development, reducing delays during regulatory review.
Learn more about our services:
https://resolvemass.ca/forced-degradation-studies/
https://resolvemass.ca/how-to-design-forced-degradation-studies/
https://resolvemass.ca/outsourcing-forced-degradation-studies-in-canada/
https://resolvemass.ca/forced-degradation-testing-service/
Contact ResolveMass Today
Conclusion
This comprehensive forced degradation study of Alectinib Hydrochloride identified four previously unknown oxidative impurities—an N-oxide, epoxide, N-hydroxy, and amide degradant. These findings confirm that Alectinib is particularly sensitive to oxidative stress and requires well-designed analytical methods to ensure ongoing stability and safety.
Such insights are essential for maintaining patient safety, manufacturing consistency, and regulatory compliance. They also support better formulation strategies and long-term quality assurance.
At ResolveMass Laboratories, we combine expert analytical chemists with advanced instrumentation to deliver clarity and confidence in impurity profiles.
For project inquiries or consultation:
👉 Contact page
FAQs on Forced Degradation & Impurity Profiling of Alectinib
Forced degradation studies evaluate how Alectinib behaves under extreme conditions to reveal its inherent stability and degradation patterns. This information helps identify impurities that may emerge during manufacturing or storage. The results support the development of stability-indicating analytical methods and ensure compliance with ICH requirements for safety and quality.
Although Alectinib remains relatively stable in acidic, basic, thermal, and photolytic environments, certain functional groups make it vulnerable to oxidation. Its tertiary amine, nitrogen-rich aromatic rings, and cyano moiety can undergo oxidative transformation. These features facilitate the formation of N-oxide, epoxide, and N-hydroxy derivatives under oxidative stress.
Characterizing unknown impurities requires an integrated analytical strategy. LC–MS/MS provides molecular weight and fragmentation data, while preparative HPLC isolates impurities as pure fractions. NMR techniques enable precise structural confirmation, and FTIR helps verify functional group changes. Together, these tools deliver a complete impurity identification framework.
The study revealed four oxidative degradation products formed under stress conditions. These included N-oxide, epoxide, N-hydroxy, and amide derivatives of Alectinib. Three impurities exhibited similar molecular mass values, indicating the presence of structural isomers produced through competing oxidative pathways.
Isolating impurities ensures that each compound is obtained in a purified state, eliminating the interference caused by mixed signals. This purity allows accurate interpretation of NMR peaks and mass spectral data. Such isolation is essential for definitive structural identification and for satisfying regulatory expectations for impurity profiling.
Impurity assessment for Alectinib must follow international guidelines covering stress studies, photostability, and impurity reporting. Key documents include ICH Q1A(R2), Q1B, Q3A(R2), and Q3B(R2). These guidelines help determine when impurities need to be identified, characterized, controlled, and documented.
The amide impurity results from oxidative conversion of the cyano group, which is confirmed through corresponding IR and NMR spectral shifts. Its detection suggests that cyano-to-amide transformation may occur under prolonged oxidative exposure. Recognizing this pathway helps anticipate potential stability issues during product storage.
Oxidative impurities may alter the potency, safety profile, or metabolic behavior of Alectinib. Identifying and controlling these degradants ensures that the drug maintains consistent performance throughout its shelf life. Proper characterization also supports safe impurity limits and prevents unexpected degradation in real-world conditions.
Finding structural isomers indicates that multiple oxidation routes can act on the Alectinib molecule simultaneously. These parallel pathways yield products with identical masses but distinct structures. This highlights the need for high-resolution separation methods to ensure accurate detection and quantification of each degradant.
References
- Parmar, R., Rajput, S., & Mohan, A. (2021). Identification, isolation, and structure elucidation of novel forced degradation products of alectinib hydrochloride. Sep Sci Plus, 4, 174–184. https://doi.org/10.1002/sscp.202000104
- Somase, K., & Rishipathak, D. (2022). A review on forced degradation studies, stability indicating method and stability profile of few antiviral drugs. Journal of Pharmaceutical Negative Results, 13(Special Issue 1), 1315–1330. https://doi.org/10.47750/pnr.2022.13.S01.158
- Zelesky, T., & Co-authors. (2023). Pharmaceutical forced degradation (stress testing) of drug substances and drug products. Journal of Pharmaceutical Sciences. Advance online publication. https://doi.org/10.1016/S0022-3549(23)00362-3


