Introduction: The Growing Importance of PEG Hydrogels in Drug Delivery
Poly(ethylene glycol) (PEG) hydrogels have emerged as a leading platform for controlled release of therapeutics. The formulation of poly(ethylene glycol) hydrogels for drug delivery delivers unmatched potential in controlled release, biocompatibility, and site-specific targeting. At ResolveMass Laboratories Inc., Our custom polymer synthesis service designs PEG-based polymers with specific release profiles. Through years of hands-on experience and in-house R&D, we’ve come to truly understand what makes the formulation of poly(ethylene glycol) hydrogels for drug delivery such a game-changer.
What are Poly(ethylene glycol) (PEG) Hydrogels?
PEG hydrogels are fundamentally formed when PEG macromers, such as PEG diacrylate or multi-arm PEG, undergo crosslinking in an aqueous medium. Upon swelling, they create a three-dimensional network capable of encapsulating drugs. When they swell, a three-dimensional network is created that can encapsulate drugs. The ability to modify chemical and mechanical properties is a cornerstone of PEG gel composition. Since PEG hydrogels are inert to the majority of tissues, they are frequently referred to as “bioinert.” Nevertheless, bioactive motifs can be added to them. For example, to encourage cell integration and enzyme-mediated breakdown, researchers add extracellular matrix proteins or peptides (such as collagen or laminin) to the network.This hybrid formulation allows the hydrogel to gradually degrade in vivo, releasing the drug as the matrix is broken down.
While shorter chains and tighter networks slow release, longer PEG chains or lower crosslink density result in larger pores and faster drug diffusion. PEG is frequently end-functionalized by chemists to facilitate a variety of crosslinking reactions (e.g. acrylate, thiol, and maleimide groups).
Key Considerations in the Formulation of Poly(ethylene glycol) Hydrogels for Drug Delivery
- Polymer Architecture: PEG comes in various architectures (linear, 4-arm, 8-arm, block copolymers). Multi-arm PEG can produce more highly crosslinked networks. Block copolymers (e.g. PEG–polyester–PEG) can self-assemble into micelles that further crosslink. Adjusting architecture allows designers to tailor network density and degradation.
- Molecular Weight and Crosslink Density: As noted by Lieberthal and Kao, higher PEG molecular weight and lower crosslinking create larger pores, accelerating diffusion. Conversely, short PEG or tighter networks slow drug release. Thus, choosing PEG MW is a primary control knob in formulation.
- Crosslinking Chemistry: PEG can be functionalized with reactive groups enabling diverse gelation methods. Common approaches include free-radical photopolymerization of acrylate-PEG, Michael addition between thiol-PEG and vinyl-PEG, enzymatic crosslinking, or “click” reactions like thiol-ene and Diels–Alder.
- Gelation Mechanism: Formulations may rely on external triggers. UV light or visible photo initiators can rapidly cure PEG acrylates. Alternatively, gels can form in situ via chemical crosslinkers, pH shifts, or temperature changes. Injectable formulations often use room-temperature gelling so they solidify upon injection or exposure to body conditions.
- Drug Loading and Release Mechanism: The nature of the drug (small molecule, protein, nanoparticle) influences loading strategy. Hydrophilic drugs may be simply mixed into the PEG solution before gelation. Larger biomolecules often require gentle gelation conditions to maintain activity. In all cases, release is typically diffusion-controlled: the drug slowly travels through the aqueous pores of the swollen gel. Degradation can also contribute: incorporating cleavable linkers (e.g. enzyme-sensitive peptides or hydrolytically labile bonds) adds an additional release mechanism.
- Bioactive Additives: To improve integration with tissue or add functionality, PEG gels may include secondary polymers or nanoparticles. For example, adding collagen or hyaluronic acid creates an interpenetrating network that enhances cell attachment and can make the hydrogel partially degradable by native enzymes. This is critical in applications where the gel must eventually disappear as the drug is released.
- Stability and Storage: To ensure reliable performance, PEG hydrogel precursors must remain chemically stable until the moment of use.Exposure to moisture, light, or heat can trigger unwanted: Premature crosslinking (e.g., residual acrylate reactions in PEGDA). Hydrolytic degradation (e.g., ester cleavage in PEG–polyester copolymers).
Key Properties Beneficial for Drug Delivery
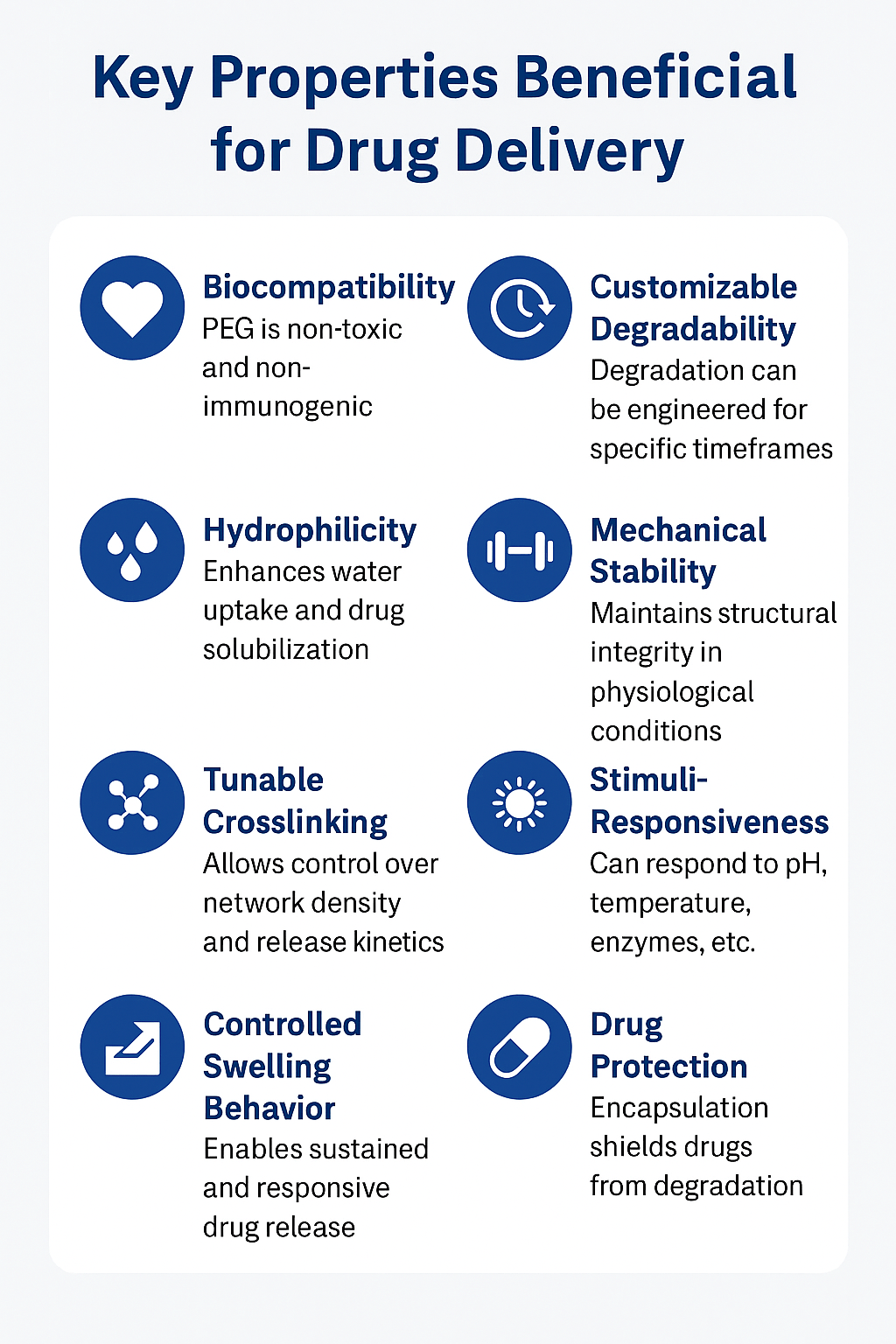
Discover more about our approach to hydrogel design at ResolveMass Custom Polymer Synthesis.
Innovations in the Formulation of Poly(ethylene glycol) Hydrogels for Drug Delivery
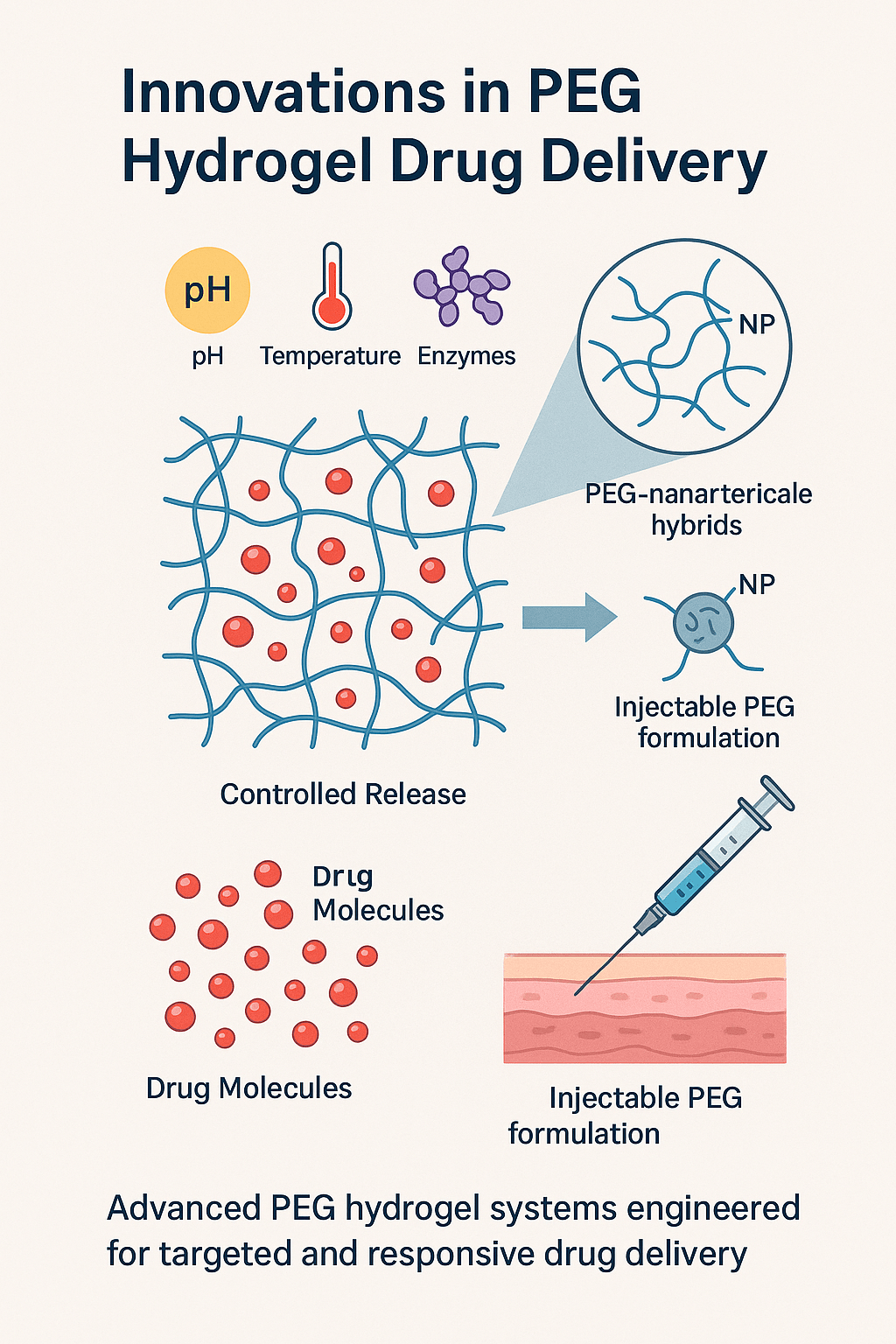
1. Smart Hydrogels with Stimuli-Responsive Behaviour
Our recent formulations integrate pH, temperature, and enzyme-responsive functionalities into PEG-based matrices. This allows for real-time responsiveness in complex physiological environments.
2. PEG Hydrogel Nanocomposites
Nanoparticle-laden PEG hydrogels are a breakthrough in enhancing drug loading efficiency and stability. Our custom nanocomposite hydrogels demonstrate sustained delivery of hydrophobic and macromolecular drugs.
3. Injectable PEG Hydrogels
We have developed thermosensitive and shear-thinning injectable PEG systems ideal for localized delivery of anticancer agents and biologics. These hydrogels conform to the site of administration and then solidify for controlled release.
Explore our proprietary research at this resource.
Want to learn more? Don’t miss our related article.
Future Directions in the Formulation of Poly(ethylene glycol) Hydrogels for Drug Delivery
1. Stimuli-Responsive (“Smart”) Hydrogels
Stimuli-responsive mechanisms for on-demand drug release will be progressively included into future PEG hydrogels, improving therapeutic precision.
Key Stimuli & Mechanisms:
- pH-Sensitive Hydrogels
- Take advantage of pH changes in disease areas, such as infected wounds and tumour microenvironments.
- Example: PEG-polyester hydrogels release medications locally when they break down more quickly in acidic environments.
- Temperature-Responsive Hydrogels
- Injectable depot formulations are made possible by thermosensitive PEG-PLGA hydrogels, which gel at body temperature.
- Enzyme-Triggered Degradation
- MMP-sensitive PEG hydrogels degrade in response to disease-specific enzymes (e.g., matrix metalloproteinases in cancer).
- Redox-Responsive Systems
- Use disulphide bonds for drug release in high glutathione (GSH) environments (e.g., intracellular tumor sites).
2. Advanced Biofunctionalization for Targeted Delivery
Future PEG hydrogels will integrate bioactive ligands to enhance cell-specific targeting and tissue integration.
Key Approaches:
- Peptide-Modified PEG Hydrogels
- RGD peptides enhance tissue regeneration by promoting cell adhesion.
- Antibody-Conjugated Hydrogels
- Anti-EGFR PEG hydrogels for administration of drugs targeted at particular tumours
- Hyaluronic Acid (HA) Hybrids
- In osteoarthritis, HA-PEG hydrogels improve cartilage targeting.
3. Injectable and 3D-Printed Hydrogel
Minimally invasive delivery and patient-specific customisation will be the main goals of next-generation PEG hydrogels.
Key Innovations:
- Shear-Thinning Injectable Hydrogels
- PEG-nanoclay or PEG-alginate hydrogels gel rapidly in situ and flow under shear stress (such as syringe injection).
- 3D-Bioprinted PEG Hydrogels
- Photocrosslinkable PEGDA bioinks enable organ-specific drug depots (e.g., printed liver patches for sustained drug release).
- Microfluidic-Assisted Hydrogel Microparticles
- PEG microgels are produced in large quantities for oral or pulmonary medication administration.
4. Nanocomposite and Hybrid Hydrogels
Incorporating nanomaterials enhances PEG hydrogel functionality for multimodal therapy.
Key Nanocomposite Strategies:
- PEG-Gold Nanoparticle (AuNP) Hybrids
- AuNPs enable photothermal-triggered drug release in cancer therapy.
- PEG-Carbon Nanotube (CNT) Networks
- Improve mechanical strength and electrically controlled drug release .
- PEG-Hydroxyapatite (HAp) Composites
- Promote bone regeneration while delivering osteogenic drugs (e.g., BMP-2).
5. Long-Acting and Biodegradable Formulations
Future PEG hydrogels must balance long-term stability with controlled biodegradability to avoid accumulation.
Key Advances:
- Tunable Degradation via Crosslinking
- Hydrolytic (ester-based) vs. enzymatic (protease-sensitive) degradation.
- Self-Healing PEG Hydrogels
- After injection, hydrogel can be repaired thanks to dynamic covalent bonds like Diels-Alder and Schiff base.
- PEG-Polylactide (PLA) Blends
- Drug release over several months due to slow degradation (e.g., hormone therapy).
6. AI-Driven Hydrogel Design
PEG hydrogel optimisation will be accelerated by computer modelling and machine learning.
Applications:
- Predicting Drug Release Kinetics
- AI models analyze crosslinking density, porosity, and drug diffusion.
- High-Throughput Screening
- Robotics + AI test thousands of PEG copolymer combinations for optimal properties.
Real-Time Case Study: Injectable PEG Hydrogel for Post-Surgical Pain Management
Background: A mid-sized hospital collaborated with ResolveMass Laboratories Inc. to implement a hydrogel-based localized drug delivery system to manage post-operative pain in orthopedic surgery.
Formulation Strategy: We utilized a PEG-based injectable hydrogel containing bupivacaine with temperature-sensitive gelling properties.
Results:
- Sustained drug release for up to 72 hours
- 45% reduction in opioid usage post-surgery
- 35% faster patient mobilization time
Conclusion: This project highlighted how the formulation of poly(ethylene glycol) hydrogels for drug delivery can directly improve patient outcomes and minimize systemic drug exposure.
Why Choose ResolveMass Laboratories Inc.?
ResolveMass Laboratories Inc. has a proven track record in custom hydrogel design, analytical characterization, and scalable synthesis. Our team consists of polymer scientists, pharmaceutical technologists, and biomedical engineers who bring unmatched experience and expertise to every project. Our custom polymer synthesis and innovative solutions are trusted by biotech companies and academic partners alike.
How We Ensure Quality and Trust
We follow Good Manufacturing Practice (GMP) standards, ISO-certified analytical validations, and FDA-aligned documentation processes. We also provide complete transparency in every stage of formulation, ensuring authoritativeness and trustworthiness in our work.
Conclusion: Formulation of Poly(ethylene glycol) Hydrogels for Drug Delivery
The formulation of poly(ethylene glycol) hydrogels for drug delivery stands at the frontier of pharmaceutical and biomedical innovation. With continued research, cross-disciplinary collaboration, and real-world testing, PEG hydrogels will become even more integral to personalized and precision medicine. Partner with ResolveMass Laboratories Inc. to experience innovation you can trust.
For project collaboration and inquiries, connect with us at our Contact Page, or explore our advanced capabilities in custom polymer synthesis.
FAQs: Formulation of Poly(ethylene glycol) Hydrogels for Drug Delivery
1. What is the role of PEG hydrogels in drug delivery?
PEG hydrogels serve as carriers for controlled drug release. Their structure allows precise release of drugs over time, minimizing side effects and improving therapeutic efficacy.
2. How are PEG hydrogels synthesized for drug delivery?
Typically, PEG chains are crosslinked using physical or chemical methods like UV irradiation, enzyme-catalyzed reactions, or click chemistry to form hydrogels suitable for drug loading.
3. Are PEG hydrogels safe for human use?
Yes. PEG is FDA-approved and widely used due to its biocompatibility and low toxicity. When formulated correctly, PEG hydrogels are safe for in vivo applications.
4. Can PEG hydrogels deliver both hydrophilic and hydrophobic drugs?
Yes. Advanced PEG hydrogel systems, including nanocomposites, can encapsulate both hydrophilic and hydrophobic drugs by modifying the polymer matrix.
5. What makes PEG hydrogels better than traditional drug carriers?
They offer controlled release, biocompatibility, and the ability to target specific sites, which reduces systemic side effects compared to traditional carriers.
6. What are the mechanisms of drug release in PEG hydrogels?
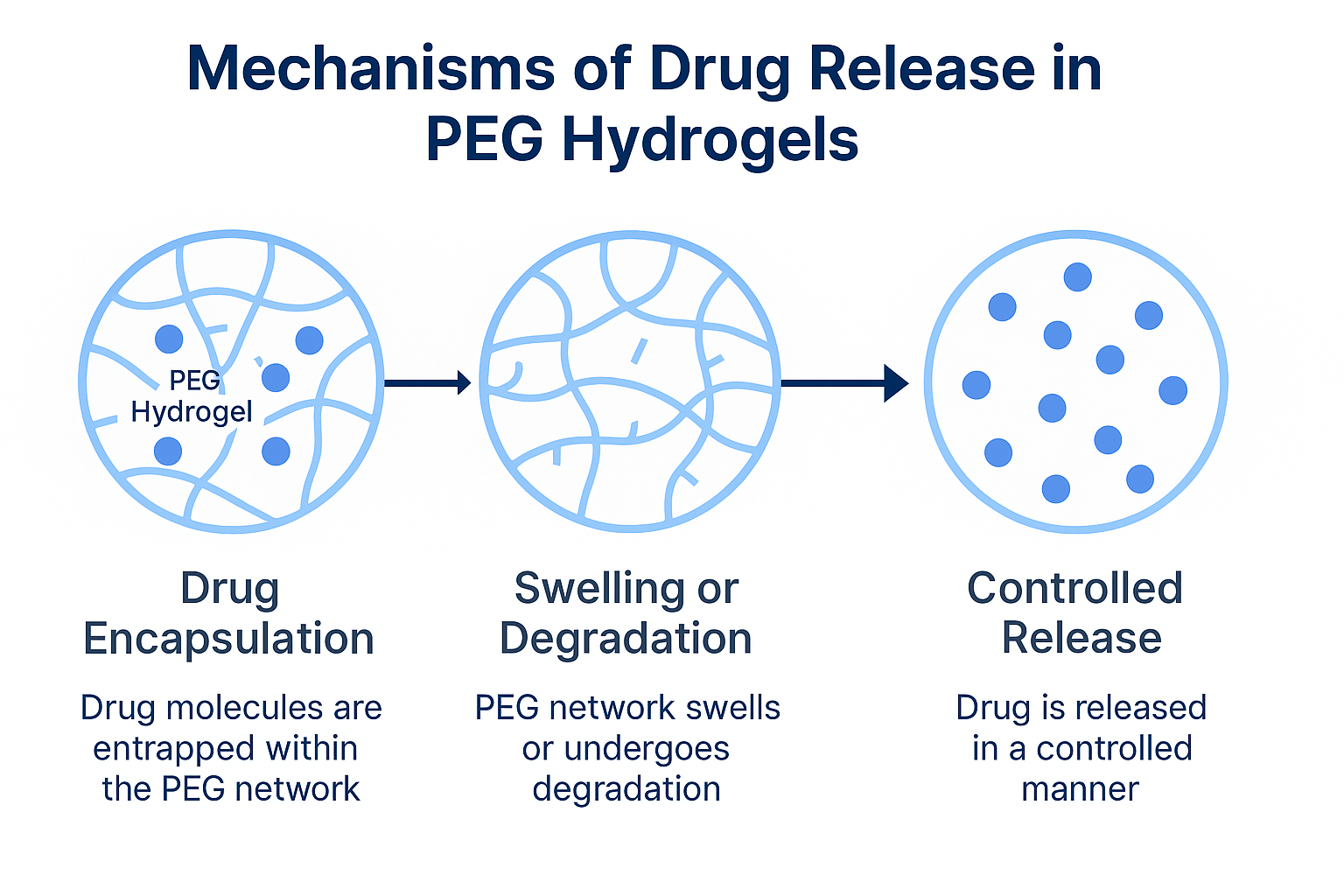
Depending on formulation, drug release can range from a few hours to several weeks. This is tunable by adjusting crosslink density and polymer architecture.
7. Can PEG hydrogels be injected?
Yes. Injectable PEG hydrogels are formulated to gel at body temperature or in response to shear force, making them ideal for minimally invasive applications.
8. What drugs are typically delivered using PEG hydrogels?
Anticancer agents, antibiotics, analgesics, and biologics like proteins and RNA-based drugs are commonly delivered using PEG hydrogels.
9. Are PEG hydrogels suitable for personalized medicine?
Absolutely. PEG hydrogels can be tailored to individual patient profiles and used with AI to predict optimal drug release patterns.
10. Where can I get custom PEG hydrogel formulations?
ResolveMass Laboratories Inc. offers custom synthesis of PEG hydrogels tailored for drug delivery. Reach out through our Contact Page to begin your project.
Contact Us for Custom Solutions
ResolveMass Laboratories Inc.: Experience, Expertise, and Trust You Can Count On
ResolveMass Laboratories Inc. is a leading name in nitrosamine testing across the United States and Canada. With over a decade of experience, our PhD-level scientists specialize in Mass Spectrometry and nitrosamine impurity chemistry. We offer complete in-house solutions, including risk assessment, confirmatory analysis, regulatory documentation, and expert consultation. As one of the few Canadian CROs, we also provide custom synthesis of rare nitrosamine impurities unavailable elsewhere. Our commitment to advanced technology and regulatory compliance ensures accurate results and trusted partnerships. Choose ResolveMass Laboratories for precise and transparent nitrosamine testing services.
Ready to Get Started?
📩 Contact our expert team
📞 Request a quote for method development
📅 Book a consultation with our scientists
🧪 Submit your sample for testing
References
- Peppas, N. A., & Hoffman, A. S. (2020). Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials, 32(18), 1905312. https://doi.org/10.1002/adma.201905312
- Zhu, J. (2010). Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials, 31(17), 4639–4656. https://doi.org/10.1016/j.biomaterials.2010.02.044
- Qiu, Y., & Park, K. (2012). Environment-sensitive hydrogels for drug delivery. Advanced Drug Delivery Reviews, 64, 49–60. https://doi.org/10.1016/j.addr.2012.09.024
- Li, J., & Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nature Reviews Materials, 1(12), 16071. https://doi.org/10.1038/natrevmats.2016.71
- Buwalda, S. J., Boere, K. W. M., Dijkstra, P. J., Feijen, J., Vermonden, T., & Hennink, W. E. (2014). Hydrogels in a historical perspective: From simple networks to smart materials. Journal of Controlled Release, 190, 254–273. https://doi.org/10.1016/j.jconrel.2014.03.003
- Nuttelman, C. R., Tripodi, M. C., & Anseth, K. S. (2005). In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. Tissue Engineering, 11(5–6), 1004–1013. https://doi.org/10.1089/ten.2005.11.1004
🧫 How to Modify the Degradation Rate of PEG Hydrogels for Sustained Drug Release
🧠 Why It’s Important:
The degradation rate of Poly(ethylene glycol) hydrogels is a critical factor in designing controlled and sustained drug delivery systems. Whether you’re targeting short-term wound healing or long-term cancer therapy, tuning the degradation profile helps maintain therapeutic drug levels over the required time.
This section walks you through how to tailor PEG hydrogel degradation rates for specific drug delivery goals.
🔧 Step-by-Step: Tuning Degradation Rates of PEG Hydrogels
Step 1: Choose the Right PEG Molecular Weight
- Lower MW (e.g., 1–4 kDa): Faster degradation due to shorter chains and lower crosslink density.
- Higher MW (e.g., 6–10 kDa): Slower degradation due to tighter networks and larger mesh sizes.
Step 2: Adjust the Crosslinker Concentration
- Increase PEGDA or PEGMA content to reduce degradation (forms tighter mesh).
- Decrease crosslinker for a looser matrix, enhancing degradation and drug diffusion.
Step 3: Incorporate Hydrolytically Labile Linkers
- Use ester, anhydride, or acetal-based bonds that break down in aqueous environments.
- Ideal for biodegradable systems that dissolve in the body after drug release.
Step 4: Introduce Enzyme-Sensitive Sites
- Add peptide sequences cleavable by matrix metalloproteinases (MMPs), abundant in tumor/wound microenvironments.
- Enables site-specific degradation triggered by disease-state enzymes.
Step 5: Modify pH Sensitivity
- Add acid-labile linkers like hydrazone or imine groups for faster degradation in acidic conditions (like tumors or inflamed tissue).
- Use basic linkers for intestinal pH-responsiveness.
Step 6: Blend with Co-polymers
- Blend PEG with PLGA, PCL, or gelatin to add bio-resorbable features and further tune degradation.
- Allows multi-phase degradation and staged drug release.
Step 7: Perform In Vitro Degradation Studies
- Place hydrogel in PBS or simulated body fluid at 37°C.
- Monitor weight loss, swelling index, and mechanical strength over time.
- Analyze degradation products for safety using LC-MS or FTIR.
PK and TK Bioanalysis: What Regulators Expect from CRO Data
Introduction: In modern drug development, PK TK bioanalysis is one of the most scrutinized scientific…
How Bioanalytical Data Supports IND-Enabling Studies
Introduction: IND enabling Bioanalytical Studies are the scientific proof that a drug can be safely…
Where to Buy Deuterated Benzene-d6 for OLED Material Research?
Introduction: If you are planning to Buy Deuterated Benzene-d6 for OLED material research, it is…
High-Purity Deuterated Benzene-d6 for OLED Applications: Specifications, Purity & Bulk Supply
Introduction: High-purity Deuterated Benzene-d6 for OLED Application plays a key role in the development of…
Case Study: Identifying Unknown Impurities using High-Resolution Mass Spectrometry
Introduction: Identifying Unknown Impurities by HRMS is one of the most powerful analytical approaches in…
Advanced Technical Strategies in the Contract Manufacturing of Peptide-Oligonucleotide Conjugates
Introduction: Peptide Oligonucleotide Conjugate Manufacturing is a highly specialized process that combines peptide chemistry with…