Introduction
GC-MS method development is a critical cornerstone of pharmaceutical quality control, impurity profiling, and regulatory compliance. As pharmaceutical companies face increasingly stringent regulatory requirements and complex analytical challenges, selecting the right analytical laboratory partner for GC-MS method development and validation becomes paramount to product success and market approval.
At ResolveMass Laboratories Inc., we understand that pharmaceutical companies require more than just accurate results—they need a strategic partner who can navigate the complexities of method development, validation protocols, and regulatory submissions. With over a decade of experience serving the pharmaceutical industry, our team of analytical chemists and regulatory specialists has developed and validated hundreds of GC-MS methods for diverse applications, from residual solvent analysis to impurity profiling and stability studies.
To understand the fundamentals of this technique, refer to the detailed overview of Gas Chromatography Mass Spectrometry and its working principle of GC-MS:
- https://resolvemass.ca/gas-chromatography-mass-spectrometry/
- https://resolvemass.ca/working-principle-of-gc-ms/
This guide explores the essential factors pharmaceutical companies should consider when evaluating GC-MS method development service providers, ensuring your analytical methods meet both scientific rigor and regulatory standards.
At ResolveMass Laboratories Inc., we provide comprehensive GC-MS analysis services that combine regulatory expertise, advanced instrumentation, and scientifically rigorous methodologies to deliver reliable, submission-ready data:
Summary
When selecting a partner for GC-MS method development and validation, pharmaceutical companies need a laboratory that combines cutting-edge technology with proven regulatory expertise. This comprehensive guide outlines the critical factors pharma companies should evaluate, including:
- Regulatory Compliance: FDA, ICH, and USP guideline adherence for method validation
- Technical Capabilities: Advanced instrumentation and experienced chromatography specialists
- Method Development Expertise: Systematic approach to optimization and troubleshooting
- Quality Assurance: Robust documentation and quality management systems
- Turnaround Time: Efficient project management without compromising quality
- Cost-Effectiveness: Transparent pricing and value-driven services
- Industry Experience: Track record in pharmaceutical analysis and submissions
- Communication: Regular updates and technical support throughout the project
For specialized regulatory-focused solutions, explore our dedicated GC-MS Method Development Service:
1: Understanding GC-MS Method Development in Pharmaceutical Context
GC-MS method development is the systematic process of creating validated analytical procedures to identify and quantify volatile and semi-volatile compounds in pharmaceutical products. This involves optimizing chromatographic conditions, mass spectrometry parameters, and sample preparation techniques to achieve reliable, reproducible results that meet regulatory requirements.
A detailed overview of GC-MS applications is available here:
The pharmaceutical industry relies on GC-MS for multiple critical applications:
- Residual solvent analysis in compliance with ICH Q3C
https://resolvemass.ca/gcms-residual-solvent-analysis-what-you-must-know/ - Genotoxic impurity detection and quantification
- Extractables and leachables studies
- Active pharmaceutical ingredient (API) purity assessment
- Stability-indicating method development
- Degradation product identification
- Process impurity profiling
GC-MS is also widely used for complex botanical and plant extract characterization, supporting pharmaceutical, nutraceutical, and natural health product development:
- https://resolvemass.ca/gcms-analysis-of-plant-extract/
- https://resolvemass.ca/lcms-analysis-of-plant-extract/
Why Method Development Expertise Matters
Poor GC-MS method development can lead to costly project delays, regulatory rejections, and even product recalls. A well-developed method must demonstrate:
- Specificity: Ability to distinguish the target analyte from matrix components
- Sensitivity: Detection limits appropriate for intended use
- Linearity: Proportional response across the working range
- Accuracy: Results close to true values
- Precision: Reproducibility under varied conditions
- Robustness: Performance stability under slight parameter variations

2: Key Factors Pharma Companies Should Evaluate
1. Regulatory Expertise and Compliance
The laboratory must demonstrate comprehensive knowledge of FDA, ICH, EMA, and USP guidelines for method validation. This ensures your analytical methods will withstand regulatory scrutiny during submissions.
ResolveMass Laboratories Inc. supports regulatory-compliant GC-MS testing for pharmaceutical submissions, quality control, and pesticide residue studies:
ResolveMass Laboratories Inc. maintains strict adherence to:
- ICH Q2(R1) guidelines for analytical validation
- FDA guidance documents for pharmaceutical analysis
- USP General Chapters <621> and <467>
- GLP and GMP compliance standards
- 21 CFR Part 11 for electronic records
Our validation packages include complete documentation packages ready for regulatory submission, including:
| Documentation Component | Purpose | Regulatory Reference |
|---|---|---|
| Validation Protocol | Defines acceptance criteria and testing plan | ICH Q2(R1) |
| Method Parameters | Specificity, linearity, accuracy, precision | FDA Guidelines |
| System Suitability | Ensures ongoing method performance | USP <621> |
| Validation Report | Summarizes all results and conclusions | ICH/FDA Requirements |
| Analytical Procedures | Step-by-step SOPs for routine use | GMP Compliance |
2. Advanced Instrumentation and Technology
The laboratory should possess state-of-the-art GC-MS equipment with multiple detector options and software capabilities. Modern instrumentation significantly impacts method sensitivity, resolution, and data quality.
Critical equipment considerations include:
- High-resolution mass spectrometry (HRMS) for accurate mass determination
- Triple quadrupole MS/MS for targeted quantification and enhanced selectivity
- Headspace and thermal desorption capabilities for volatile analysis
- Multiple ionization modes (EI, CI, APCI) for diverse compound classes
- Advanced data processing software for metabolite identification and quantification
At ResolveMass Laboratories Inc., our instrumentation suite includes the latest generation GC-MS/MS systems with femtogram-level sensitivity, enabling detection of trace impurities that older instruments might miss.
Our regional service capabilities include:
- GC-MS analysis in Montreal
https://resolvemass.ca/gcms-analysis-in-montreal/ - Why ResolveMass is the preferred GC-MS laboratory in Montreal, Canada
https://resolvemass.ca/gcms-analysis-in-montreal-canada-why-resolvemass-laboratories-inc-is-your-best-choice/ - GC-MS analysis services in the United States
https://resolvemass.ca/gcms-analysis-in-united-states/
These capabilities ensure consistent analytical performance for multinational development programs and regulatory submissions.
3. Experienced Method Development Team
A laboratory’s scientific team should include Ph.D.-level analytical chemists with extensive pharmaceutical GC-MS experience. The complexity of GC-MS method development demands both theoretical knowledge and practical troubleshooting skills.
Look for teams with:
- Published research in analytical chemistry journals
- Regulatory submission experience
- Expertise in diverse pharmaceutical matrices
- Problem-solving capabilities for complex separations
- Knowledge of emerging compounds and novel dosage forms
Our scientists at ResolveMass Laboratories Inc. average 15+ years of pharmaceutical analytical experience, having successfully developed methods for over 200 pharmaceutical compounds across all major therapeutic categories.
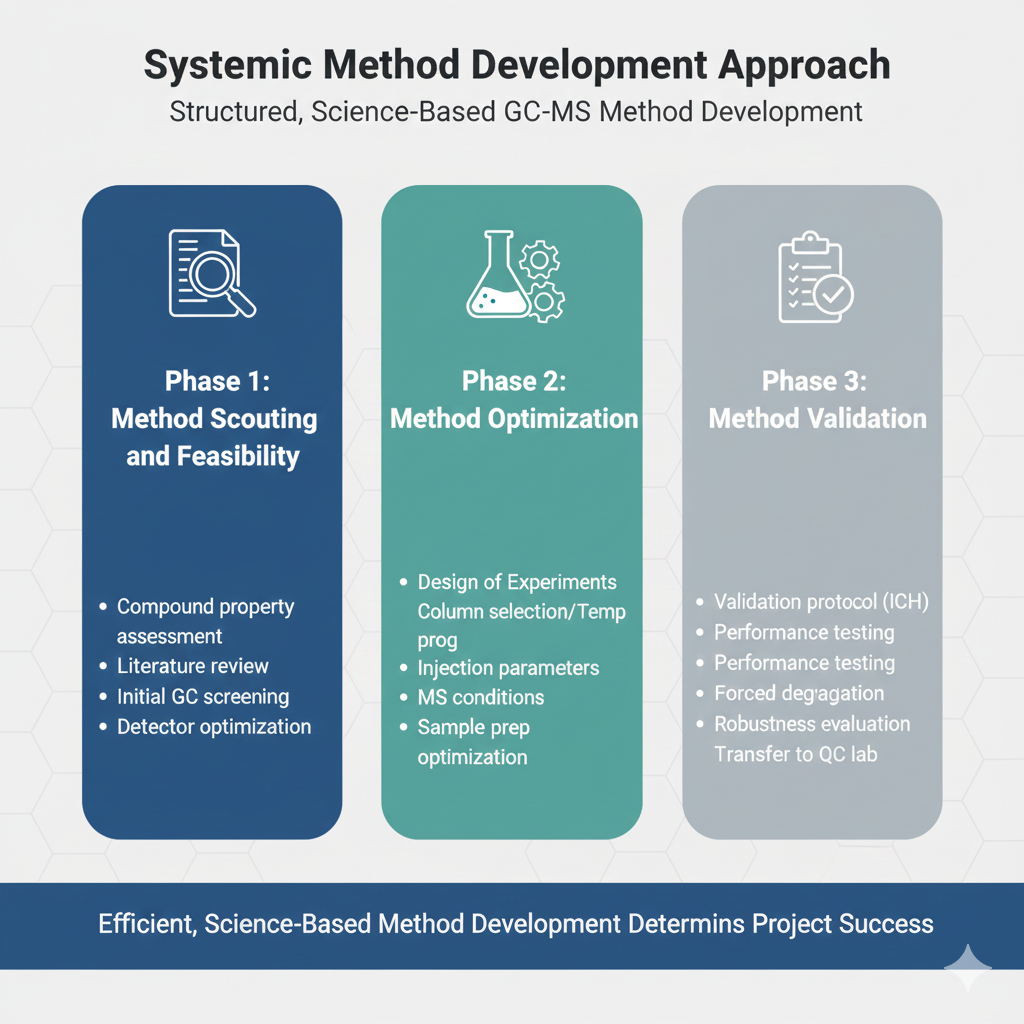
4. Systematic Method Development Approach
The laboratory should follow a structured, science-based approach to GC-MS method development rather than trial-and-error experimentation. An efficient method development process includes:
Phase 1: Method Scouting and Feasibility
- Compound physicochemical property assessment
- Literature review and method evaluation
- Initial screening of chromatographic conditions
- Detector optimization and mass spectral library matching
- https://resolvemass.ca/gcms-analysis-service-2/
Phase 2: Method Optimization
- Design of Experiments (DOE) for parameter screening
- Column selection and temperature programming
- Injection parameters and split ratios
- MS conditions (scan range, dwell times, collision energies)
- Sample preparation optimization
Phase 3: Method Validation
- Validation protocol development per ICH guidelines
- Performance characteristic testing
- Forced degradation studies
- Robustness and ruggedness evaluation
- Transfer to QC laboratory (if required)
5. Quality Management Systems
A robust quality management system ensures consistent, reliable results throughout method development and routine analysis. Pharmaceutical companies should verify:
- ISO/IEC 17025 accreditation status
- Documented quality manual and SOPs
- Comprehensive training programs
- Regular equipment qualification (IQ/OQ/PQ)
- Proficiency testing participation
- Internal quality control procedures
- CAPA (Corrective and Preventive Action) systems
ResolveMass Laboratories Inc. maintains ISO/IEC 17025 accreditation and follows stringent quality protocols, with quarterly external audits and continuous internal quality monitoring.
6. Turnaround Time and Project Management
Efficient project management balances speed with scientific rigor, delivering validated methods without compromising quality. Inquire about:
- Typical timelines for GC-MS method development projects
- Communication protocols and progress updates
- Flexibility for expedited services
- Ability to handle multiple simultaneous projects
- Experience managing tight regulatory deadlines
| Project Phase | Typical Timeline | ResolveMass Capabilities |
|---|---|---|
| Method Development | 3-6 weeks | 2-4 weeks with express service |
| Method Validation | 2-4 weeks | 1-3 weeks (depending on complexity) |
| Report Generation | 1-2 weeks | 3-5 business days |
| Total Project | 6-12 weeks | 4-8 weeks |
7. Cost Transparency and Value
While cost is important, the lowest price often indicates compromises in expertise, equipment, or documentation quality. Evaluate:
- Detailed, itemized pricing proposals
- What’s included in quoted prices (equipment time, consumables, reporting)
- Additional fees for revisions or troubleshooting
- Volume discounts for multiple methods
- Cost comparison based on deliverables, not just price
ResolveMass Laboratories Inc. provides transparent, competitive pricing with no hidden fees. Our proposals clearly outline all costs, deliverables, and timelines before project initiation.
8. Industry Track Record and References
A proven track record in pharmaceutical GC-MS method development demonstrates competence and reliability. Request:
- Case studies from similar projects
- Client testimonials and references
- Number of regulatory submissions supported
- Success rate for method validation
- Publications and conference presentations
Our portfolio includes successful GC-MS method development projects for:
- Generic drug manufacturers requiring bioequivalence studies
- Innovative pharmaceutical companies developing novel formulations
- Contract manufacturing organizations (CMOs) needing method transfer
- Regulatory agencies requiring independent verification
9. Technical Support and Communication
Ongoing technical support and clear communication are essential throughout method development and beyond. Evaluate:
- Dedicated project manager assignment
- Regular status updates and meetings
- Technical consultation availability
- Post-validation support for method implementation
- Troubleshooting assistance during technology transfer
At ResolveMass Laboratories Inc., every client receives a dedicated project team including a Ph.D. scientist and project coordinator, ensuring seamless communication and rapid response to questions or concerns.
10. Flexibility and Customization
Every pharmaceutical product presents unique analytical challenges requiring customized method development approaches. Look for laboratories that:
- Adapt protocols to specific product requirements
- Handle non-standard sample matrices
- Accommodate special regulatory requirements
- Develop methods for emerging technologies (gene therapies, biologics)
- Provide method modification services as products evolve
3: Red Flags to Avoid When Selecting a GC-MS Service Provider
When evaluating laboratories for GC-MS method development, be cautious of:
- Vague validation proposals lacking specific protocols or acceptance criteria
- Outdated instrumentation that may not meet current sensitivity requirements
- Limited regulatory knowledge or absence of submission experience
- Poor documentation practices that could jeopardize regulatory acceptance
- Unrealistic timelines that suggest rushed or inadequate validation
- Reluctance to provide references or case studies from pharma clients
- Lack of quality certifications (ISO accreditation, regulatory inspections)
4: The ResolveMass Laboratories Inc. Advantage
- ResolveMass Laboratories Inc. has established itself as a trusted partner for pharmaceutical GC-MS method development through:
- Comprehensive Expertise: Our team includes analytical chemists specializing in pharmaceutical analysis, with advanced degrees and decades of combined experience in GC-MS method development, validation, and regulatory submissions.
- Cutting-Edge Technology: We invest continually in the latest GC-MS instrumentation, including high-resolution systems and advanced software platforms, ensuring your methods leverage the most sensitive and selective technologies available.
- Regulatory Excellence: With hundreds of successful regulatory submissions supporting our clients’ IND, NDA, and ANDA applications, we understand exactly what regulatory agencies expect from method validation documentation.
- Quality Commitment: Our ISO/IEC 17025 accreditation and robust quality management systems ensure every GC-MS method development project meets the highest scientific and regulatory standards.
- Client-Centric Approach: We partner with you from initial consultation through method implementation, providing transparent communication, flexible solutions, and responsive technical support throughout your project lifecycle.
- Proven Results: Our methods have supported over 150 successful regulatory submissions across multiple therapeutic areas, demonstrating our capability to deliver validated, submission-ready analytical procedures.
5: Making the Right Choice for Your GC-MS Method Development Needs
Selecting the right analytical laboratory for GC-MS method development and validation requires careful evaluation of technical capabilities, regulatory expertise, quality systems, and industry experience. The decision impacts not only your immediate analytical needs but also long-term regulatory success and product quality assurance.
By partnering with a laboratory that demonstrates comprehensive expertise in GC-MS method development, maintains state-of-the-art instrumentation, follows systematic development approaches, and prioritizes regulatory compliance, pharmaceutical companies can ensure their analytical methods will withstand scrutiny and support successful product commercialization.
Conclusion
GC-MS method development represents a critical investment in pharmaceutical product quality and regulatory compliance. The right analytical laboratory partner brings more than technical capabilities—they provide strategic guidance, regulatory insight, and validated methods that accelerate your path to market while ensuring patient safety and product quality.
When evaluating service providers for GC-MS method development, prioritize laboratories that demonstrate deep pharmaceutical expertise, maintain cutting-edge instrumentation, follow rigorous quality standards, and have proven success supporting regulatory submissions. Don’t compromise on factors like documentation quality, communication, or regulatory knowledge in pursuit of lower costs—these investments pay dividends through faster approvals and reduced regulatory risk.
ResolveMass Laboratories Inc. stands ready to support your GC-MS method development and validation needs with comprehensive expertise, advanced technology, and unwavering commitment to quality. Our team of analytical specialists has successfully developed and validated hundreds of GC-MS methods for pharmaceutical applications, supporting clients through every stage from early development to commercial manufacturing.
FAQs on GC-MS Method Development & Validation Services
GC-MS is preferred because it combines high chromatographic separation with highly specific mass spectral identification. It is ideal for volatile, semi-volatile, and thermally stable compounds, making it indispensable for residual solvents, genotoxic impurities, extractables & leachables, and impurity profiling. The technique offers high sensitivity, excellent selectivity, and regulatory acceptance, especially for trace-level quantification.
GC-MS is widely used for:
-Residual solvent testing (ICH Q3C)
-Genotoxic impurity analysis
-Nitrosamine detection
-Extractables and leachables (E&L)
-Impurity profiling
-Forced degradation volatile products
Its ability to detect compounds at ppm to ppb levels makes it critical for regulatory submissions.
Sample preparation is critical and often determines method success. Techniques such as headspace sampling, SPME, liquid-liquid extraction, or derivatization are chosen based on analyte volatility and matrix complexity. Poor sample preparation can lead to matrix effects, low recovery, or false negatives, even with a well-optimized GC-MS system.
Pharma companies should assess:
-Experience with ICH, USP, EP, and FDA expectations
-Capability to develop matrix-specific methods
-Proper selection of column chemistry and ionization mode
-Robust sample preparation strategy
-Clear method transfer documentation
-A strong provider focuses on method robustness, specificity, and long-term reproducibility, not just peak separation.
GC-MS method validation follows ICH Q2(R1/R2) parameters including:
-Specificity
-Linearity and range
-Accuracy and precision
-Limit of Detection (LOD) and Limit of Quantitation (LOQ)
-Robustness
Validation must be performed using real sample matrices, not only standards, to ensure regulatory acceptance.
GC-MS data are widely accepted by global regulators due to the technique’s confirmatory power using mass spectra. Properly validated GC-MS methods support ANDA, NDA, DMF, and stability filings, ensuring compliance with FDA, EMA, and other regulatory bodies.
Reference
- Lakshmi HimaBindu M.R, Angala Parameswari S, Gopinath C.A Review on GC-MS and Method Development and Validation.https://impactfactor.org/PDF/IJPQA/4/IJPQA,Vol4,Issue3,Article3.pdf
- Viktor A Filatov, Egor A Ilin, Olesya Yu Kulyak, Elena I Kalenikova.Development and Validation of a Gas Chromatography–Mass Spectrometry Method for the Analysis of the Novel Plant-Based Substance with Antimicrobial Activity.https://pmc.ncbi.nlm.nih.gov/articles/PMC10603869/
- David Elder.GC-MS applications in pharmaceutic alanalysis.https://www.europeanpharmaceuticalreview.com/article/77425/gc-ms-applications-in-pharmaceutical-analysis/

