✅ Summary of the Article
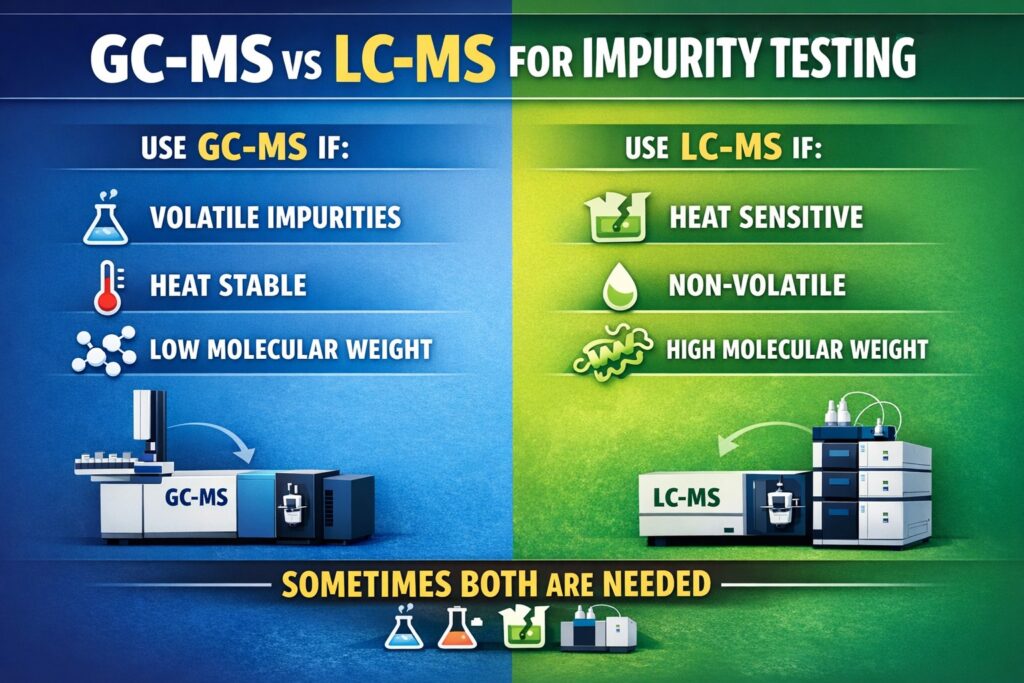
- GC-MS is ideal for volatile, thermally stable impurities with low molecular weight.
- LC-MS is the method of choice for non-volatile, thermally labile, and polar impurities.
- Regulatory agencies expect orthogonal impurity profiling—often requiring both GC-MS and LC-MS.
- Real-world applications differ by industry: pharma, environmental, food, and chemicals.
- Sample matrix, analyte properties, and regulatory guidelines dictate method choice.
- Cost, speed, sensitivity, and detection range must be considered.
- In complex impurity profiles, hyphenated or complementary analysis is recommended.
- The right choice impacts product safety, compliance, and brand trust.
Introduction: GC-MS vs LC-MS for Impurity Testing
Should you use GC-MS or LC-MS for impurity testing?
There is no one-size-fits-all answer. The choice depends on the type of impurities you’re testing for, the sample matrix, and regulatory expectations. Each method has its own strengths and limitations.
In this guide, we explore GC-MS vs LC-MS for impurity testing by using real-world examples. These practical insights will help you choose the best technique for your specific testing needs.
By selecting the right method, you can improve the quality of your results, save time on rework, and streamline regulatory approval processes.
Explore the Fundamentals: To better understand how these technologies differ, read our detailed breakdown of Gas Chromatography-Mass Spectrometry.
When GC-MS Is the Right Choice for Impurity Testing
GC-MS is best used when the impurities are volatile, heat-resistant, and low in molecular weight. These traits allow the compounds to be easily vaporized and separated without breaking down.
GC-MS also offers highly reproducible results and is trusted in routine quality control, especially in pharmaceutical and chemical industries. It is widely used when the impurities exist in the gas phase or can be easily turned into one.
In many such cases, using LC-MS would add unnecessary complexity without improving the outcome.
✅ Common Use Cases for GC-MS:
- Testing for residual solvents in drugs (as per ICH Q3C guidelines)
- Identifying volatile organic compounds (VOCs) in environmental samples
- Analyzing fragrance or flavor components in food and cosmetics
- Studying thermally stable degradation products
These applications depend on separating impurities based on volatility, which is a strong advantage of GC-MS.
Identify Volatile Compounds: If your project involves identifying specific chemical signatures, learn more about our specialized GCMS Analysis Service.
Why GC-MS Is Preferred:
GC-MS provides excellent resolution for separating volatile compounds, even in complex mixtures. It also offers access to large spectral libraries, such as NIST, that make identifying unknown compounds easier and faster.
Its consistency and reliability make GC-MS a top choice for routine testing and regulatory compliance.
Technical Insight: Understand the science behind the separation by exploring the Working Principle of GC-MS.
🔬 Real-World Example: Pharmaceutical Testing
A pharmaceutical company uses GC-MS to identify residual solvents like benzene and methanol in a drug product. These solvents are volatile and comply with ICH Q3C guidelines. Since LC-MS is not suited for detecting such volatile substances, GC-MS is the ideal method to ensure accurate results and pass regulatory inspections.
Regulatory Compliance: For specific guidance on solvent safety, check out GCMS Residual Solvent Analysis: What You Must Know.
When LC-MS Is the Right Choice for Impurity Testing
LC-MS is the best method for analyzing impurities that are heat-sensitive, non-volatile, or have high molecular weights. These compounds would break down during GC-MS analysis.
LC-MS works in the liquid phase, which protects the chemical structure and offers more precise results. It is especially useful in pharmaceuticals and biological samples where complex molecules are involved.
The technique is also effective for detecting very small amounts of impurities in difficult sample types.
✅ Common Use Cases for LC-MS:
- Detecting impurities in biologics and active pharmaceutical ingredients (APIs)
- Analyzing drug degradation products that are not volatile
- Profiling pesticide residues in complex food samples
- Studying polar metabolites in clinical and toxicology settings
These scenarios require the soft ionization and selectivity that LC-MS provides.
Plant-Based Analysis: If you are working with botanical samples that are heat-sensitive, see our specialized LCMS Analysis of Plant Extract services.
Why LC-MS Is Preferred:
LC-MS does not need high temperatures, making it perfect for fragile compounds. It also handles a wide range of analytes—from small drug molecules to large proteins—with high sensitivity.
This makes LC-MS a powerful tool for detecting trace impurities, especially in heavily regulated environments.
Optimize Your Results: Need a custom protocol for your specific analyte? Discover our GCMS Method Development Service.
🔬 Real-World Example: Biotech Testing
A biotech company uses LC-MS to identify impurities in a monoclonal antibody drug. These impurities are highly polar and sensitive to heat. GC-MS would destroy them before analysis. LC-MS allows the company to preserve the structure of these compounds and submit accurate data for regulatory review.
Key Factors in Choosing GC-MS vs LC-MS for Impurity Testing
Deciding whether to use GC-MS or LC-MS depends on several key factors. No single method works for every impurity or sample.
The table below offers a quick comparison of both methods to help guide your selection.
| Factor | GC-MS | LC-MS |
|---|---|---|
| Volatility Required | Yes | No |
| Thermal Stability | Required | Not Required |
| Analyte Polarity | Low to moderate | High |
| Sample Preparation | Often needs derivatization | Minimal |
| Sensitivity | High for volatiles | High for polar and large molecules |
| Matrix Interference | Low in clean samples | Can be reduced with right conditions |
| Common Uses | Solvents, VOCs, oils | Drugs, peptides, metabolites |
Regulatory Perspective: GC-MS vs LC-MS in Compliance Testing
Regulatory agencies like the FDA, EMA, and ICH recognize both GC-MS and LC-MS as reliable tools. The chosen method should be based on the impurity’s nature and supported by validated data.
Sometimes, both techniques are required for full coverage. Using only one may lead to gaps in impurity detection and delay product approval.
🔍 When GC-MS Is Expected:
- ICH Q3C compliance for residual solvents
- VOC analysis in environmental samples
🔍 When LC-MS Is Required:
- ICH Q3D guidelines for organic impurities
- Process-related impurities in biologics
- EU regulations for pesticide residues
Pro Tip: Submitting data from both GC-MS and LC-MS strengthens your application by offering orthogonal validation.
GC-MS vs LC-MS: A Practical Decision Tree
Choosing the right method early can help avoid re-analysis and costly delays. Here is a simple guide for selecting the best technique:
- Is the impurity volatile and heat-stable? → Choose GC-MS
- Is the impurity polar or non-volatile? → Use LC-MS
- Will heat degrade the impurity? → Use LC-MS
- Are there regulatory requirements? → Follow the specific guideline
- Is the sample matrix complex? → Use LC-MS with HPLC or SPE sample prep
Real-World Industry Applications
🧪 Pharmaceutical Manufacturing
- GC-MS: Used for residual solvents and volatile compounds
- LC-MS: Best for degradation products, genotoxic impurities, and trace components
- Combined: Ensures full impurity profiling and regulatory approval
🌱 Environmental Testing
- GC-MS: Detects VOCs in water and air
- LC-MS: Identifies emerging contaminants and endocrine disruptors
- Combined: Provides complete risk assessment data
Safety Standards: Ensure your products are contaminant-free with our Pesticide Testing Services using GC-MS in Canada.
🧃 Food Safety
- GC-MS: Analyzes flavors and fragrances
- LC-MS: Detects pesticide residues and mycotoxins
- Combined: Supports food safety and global compliance
Broad Industry Use: See how these techniques apply across various sectors in Applications of GCMS.
🧫 Chemical Industry
- GC-MS: Monitors solvents and volatile additives
- LC-MS: Detects large additives and polymer breakdown products
- Combined: Supports product consistency and quality
Hybrid Approach: Combining GC-MS and LC-MS
In many cases, using just one method leaves out important impurities. A combined strategy using both GC-MS and LC-MS ensures better impurity coverage and improved detection.
This dual approach helps detect both volatile and non-volatile compounds, providing a stronger data package for regulatory reviews.
Benefits of a Combined Approach:
- Broader impurity detection across multiple chemical properties
- Increased confidence from regulatory authorities
- Better identification of unknown compounds
🔬 Example: Cosmetics Testing
A cosmetic product is tested using both methods. GC-MS finds degradation byproducts in fragrances, while LC-MS detects harmful substances from packaging. The combined method ensures full safety evaluation.
Making the Right Choice
So, which is better—GC-MS or LC-MS for impurity testing?
- Use GC-MS when impurities are volatile, heat-stable, and have low molecular weights.
- Use LC-MS for heat-sensitive, non-volatile, or polar impurities.
- In many real-world scenarios, using both provides the most complete results.
Choose based on the impurity type, the sample matrix, and regulatory requirements. This strategy helps you meet standards efficiently and avoid delays.
Local Laboratory Expertise: Looking for a partner in the region? Find out Why ResolveMass Laboratories Inc is your best choice in Montreal, Canada.
Conclusion
Understanding the difference between GC-MS vs LC-MS for impurity testing is a crucial part of your analytical planning. The right method improves data quality, speeds up approvals, and ensures safety.
ResolveMass Laboratories Inc. uses both GC-MS and LC-MS platforms to support your impurity testing needs. We help you meet strict regulatory standards while maintaining high throughput and accuracy.
📞 Need Help Choosing Between GC-MS and LC-MS?
Let our experts guide you in building the most effective impurity testing strategy.
FAQs: GC-MS vs LC-MS for Impurity Testing
Use GC-MS when analyzing volatile, thermally stable, and low molecular weight compounds—such as solvents or small organic molecules. LC-MS is the better choice for polar, non-volatile, or heat-sensitive substances like peptides, metabolites, or drug degradation products. The selection depends on the physical and chemical nature of the impurity.
Yes, LC-MS is generally more sensitive than GC-MS, especially for detecting polar and large biomolecules at trace levels. It offers greater flexibility in handling complex or thermally unstable compounds. However, GC-MS remains highly effective for volatile and low molecular weight substances.
GC-MS is limited to compounds that are volatile and stable under high temperatures. It often requires sample derivatization for polar or non-volatile substances, which can add time and complexity. Additionally, it may not perform well with large or thermally labile molecules.
Peak purity in GC-MS refers to the assessment of whether a chromatographic peak represents a single compound or a mixture of closely eluting substances. It helps ensure accurate identification and quantification by checking for overlapping impurities or co-eluting compounds.
GC-MS is commonly used to test pharmaceuticals (e.g., residual solvents), environmental samples (e.g., VOCs in water or air), food and beverages (e.g., flavor compounds), and cosmetic products. It is ideal for quality control of volatile and thermally stable substances.
GC is preferred over LC when analyzing volatile, low molecular weight, and thermally stable compounds. It often provides faster run times, better resolution for certain analytes, and well-established methods with large spectral libraries for compound identification.
Yes, LC-MS typically offers higher sensitivity for detecting non-volatile and polar impurities, especially in complex biological or pharmaceutical samples. Its ability to analyze compounds that are unstable under heat makes it more versatile in many modern testing applications.
Reference
- Bajo-Fernández, M., Souza-Silva, É. A., Barbas, C., Rey-Stolle, M. F., & García, A. (2024). GC-MS-based metabolomics of volatile organic compounds in exhaled breath: Applications in health and disease. A review. Frontiers in Molecular Biosciences, 10, 1295955. https://doi.org/10.3389/fmolb.2023.1295955
- Jadhav, V. S., Kadam, G. L., Charde, M. S., Chakole, R. D., & Murkar, N. S. (2023). Applications of GC-MS: A review. International Journal of Scientific Development and Research, 8(6), 1209–1212. https://ijsdr.org/papers/IJSDR2306170.pdf


