
Introduction:
Identifying Unknown Impurities by HRMS is one of the most powerful analytical approaches in pharmaceutical development when unknown peaks appear during stability, process validation, or routine QC testing. Unknown impurities can delay regulatory submissions, trigger batch rejections, and pose potential safety risks if not properly characterized.
At ResolveMass Laboratories Inc., we routinely support pharmaceutical and biotech companies across North America in solving impurity-related challenges using advanced High-Resolution Mass Spectrometry (HRMS). Our expertise includes both:
- Unknown impurity isolation and structural characterization
- Regulatory-ready impurity identification investigations
Learn more about our dedicated services:
This case study demonstrates our structured, science-driven approach to Identifying Unknown Impurities by HRMS quickly, accurately, and defensibly for regulatory purposes.
Summary:
- How Identifying Unknown Impurities by HRMS helps pharmaceutical companies solve complex impurity challenges.
- Why high-resolution mass spectrometry is superior to conventional LC-MS for structural elucidation.
- A real-world case study approach used by ResolveMass Laboratories Inc.
- Step-by-step workflow for identifying unknown impurities in drug substances and formulations.
- Regulatory importance of impurity identification under ICH guidelines.
- Practical lessons for R&D, quality control, and regulatory submissions.
1: What Is High-Resolution Mass Spectrometry (HRMS)?
High-Resolution Mass Spectrometry (HRMS) is an analytical technique that measures the exact mass of ions with extremely high mass accuracy (typically <5 ppm), enabling molecular formula determination.
Unlike traditional quadrupole LC-MS systems, HRMS instruments such as Orbitrap and Time-of-Flight (TOF) systems provide:
- Accurate mass measurement
- Isotopic pattern confirmation
- Fragmentation (MS/MS) data for structural elucidation
- Detection of low-level impurities
These capabilities make HRMS the gold standard for Identifying Unknown Impurities by HRMS in pharmaceutical analysis.
How HRMS Works
HRMS separates ions based on their mass-to-charge ratio (m/z) with extremely high resolving power. This means it can distinguish between compounds that differ by very small mass differences — even fractions of a Dalton.
For example, two compounds with nearly identical nominal mass may appear the same on a low-resolution instrument. HRMS can differentiate them because it measures exact mass to four or more decimal places.
Common HRMS Instrument Platforms
Modern HRMS systems used in pharmaceutical analysis include:
- Orbitrap-based systems – Known for ultra-high mass accuracy and resolving power
- Time-of-Flight (TOF) systems – Known for fast acquisition and excellent full-scan data
Both platforms allow full-scan data acquisition, enabling retrospective data analysis — a major advantage in impurity investigations.
Key Capabilities of HRMS
HRMS offers several advantages over traditional quadrupole LC-MS systems:
- Accurate mass measurement – Enables molecular formula prediction
- Isotopic pattern confirmation – Confirms elemental composition (C, H, N, O, S, Cl, etc.)
- MS/MS fragmentation analysis – Provides structural information
- High resolving power – Separates co-eluting compounds
- Trace-level detection – Identifies low-level impurities in complex matrices
2: Why Is Identifying Unknown Impurities by HRMS Critical in Pharmaceuticals?
Unknown impurities must be identified to ensure drug safety, quality, and regulatory compliance.
Under ICH guidelines (such as Q3A and Q3B), impurities above reporting, identification, or qualification thresholds must be characterized. Failure to identify them can result in:
- Regulatory queries
- Clinical hold risks
- Delayed ANDA/NDA approvals
- Product recalls
Common Sources of Unknown Impurities:
- Process-related impurities
- Degradation products
- Residual reagents or solvents
- Packaging extractables/leachables
- Storage-induced degradation
When routine HPLC reveals an unexpected peak, Identifying Unknown Impurities by HRMS becomes essential.
Identifying Unknown Impurities by HRMS is critical because pharmaceutical products must meet strict safety, quality, and regulatory standards before they can be approved or remain on the market. Any unknown impurity above established thresholds must be structurally characterized and scientifically justified.
Regulatory agencies worldwide—including the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)—require impurity identification under guidelines such as ICH Q3A (drug substances) and ICH Q3B (drug products). These guidelines define:
- Reporting thresholds
- Identification thresholds
- Qualification thresholds
If an impurity exceeds the identification threshold and remains structurally unknown, regulatory submission may be delayed or challenged.
What Happens If Unknown Impurities Are Not Identified?
Failure to properly perform Identifying Unknown Impurities by HRMS can lead to serious regulatory and commercial consequences:
- Regulatory deficiency letters
- Clinical hold risks during development
- Delayed ANDA/NDA approvals
- Batch rejection or recall
- Additional toxicological studies
- Increased development costs
Regulatory agencies expect scientific justification supported by high-quality analytical data. HRMS provides the mass accuracy and structural confidence required to meet these expectations.
3: Case Study: Identifying Unknown Impurities using High-Resolution Mass Spectrometry
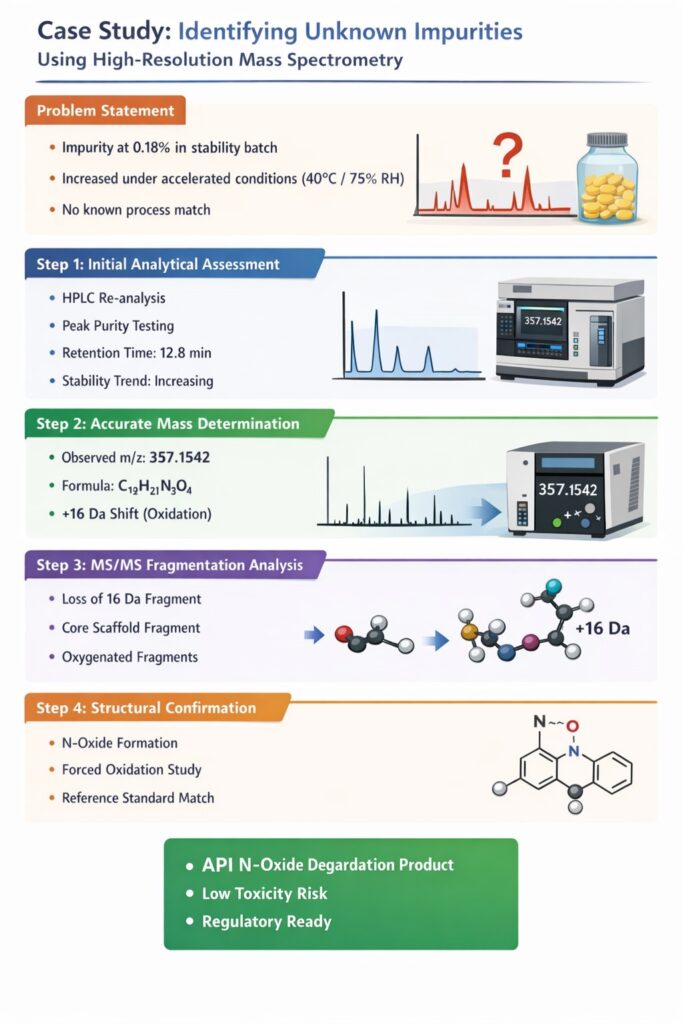
Problem Statement
A pharmaceutical manufacturer observed an unknown impurity peak at 0.18% in a stability batch of an oral solid dosage form. The impurity was:
- Not present in initial release testing
- Increased under accelerated stability (40°C/75% RH)
- Not matching any known process impurity
The sponsor required urgent structural identification for regulatory documentation.
Step 1: Initial Analytical Assessment for Identifying Unknown Impurities by HRMS
The first step in Identifying Unknown Impurities by HRMS is confirming the impurity’s presence, reproducibility, and chromatographic behavior.
Actions Taken:
- Re-analysis by HPLC with PDA detection
- Peak purity assessment
- Relative retention time determination
- Stress degradation comparison
Observations:
| Parameter | Result |
|---|---|
| Retention Time | 12.8 minutes |
| UV Max | Similar to API |
| Peak Purity | Spectrally pure |
| Stability Trend | Increasing |
The UV similarity suggested structural similarity to the API.
Step 2: Accurate Mass Determination using HRMS
Accurate mass measurement is the foundation of Identifying Unknown Impurities by HRMS because it enables molecular formula prediction.
Using LC-HRMS:
- Exact mass of impurity ion measured
- Mass accuracy: ±2 ppm
- Isotopic distribution analyzed
Results:
- Observed m/z: 357.1542
- Suggested molecular formula: C₁₉H₂₁N₃O₄
- Mass difference from API: +16 Da
A +16 Da shift often indicates oxidation.
Step 3: MS/MS Fragmentation Analysis
MS/MS fragmentation provides structural clues by breaking the molecule into diagnostic fragments.
Key fragments observed:
- Loss of 16 Da fragment
- Fragment corresponding to intact core scaffold
- Additional oxygen-related fragmentation pattern
Interpretation: The impurity was likely a mono-oxidized derivative of the API.
This step is critical in Identifying Unknown Impurities by HRMS because accurate mass alone cannot confirm structure.
Step 4: Structural Hypothesis and Mechanistic Evaluation
After fragmentation analysis, chemical reasoning is applied to propose a plausible degradation pathway.
Possible mechanisms evaluated:
- N-oxide formation
- Aromatic hydroxylation
- Side-chain oxidation
Stability data and oxidative stress studies suggested N-oxide formation under accelerated conditions.
To confirm:
- Forced oxidation study conducted
- Synthetic reference standard comparison performed
The impurity matched an N-oxide derivative of the API.
Final Identification Outcome
The unknown impurity was confirmed as:
API N-oxide degradation product
Regulatory Significance:
- Known degradation pathway
- Toxicologically low risk
- Controlled via packaging optimization
This completed the process of Identifying Unknown Impurities by HRMS with regulatory defensibility.
4: Why HRMS Is Superior for Identifying Unknown Impurities
HRMS is superior because it provides exact mass accuracy, reliable molecular formula determination, and advanced structural elucidation—making it far more powerful than conventional LC-MS for Identifying Unknown Impurities by HRMS.
When an unknown impurity appears in a chromatogram, speed and certainty matter. Conventional LC-MS can provide approximate molecular weight information, but it often lacks the resolution and accuracy required for confident structural assignment. HRMS eliminates ambiguity by delivering ppm-level mass accuracy and high resolving power.
Technical Comparison: Conventional LC-MS vs HRMS
| Feature | Conventional LC-MS | HRMS |
|---|---|---|
| Mass Accuracy | ±0.5 Da | <5 ppm |
| Formula Determination | Limited | Accurate and confident |
| Isotopic Pattern Analysis | Limited resolution | High-confidence elemental confirmation |
| Structural Elucidation | Basic fragmentation | Advanced MS/MS with high resolution |
| Regulatory Acceptance | Moderate | High for impurity characterization |
5: Practical Lessons from This Case Study
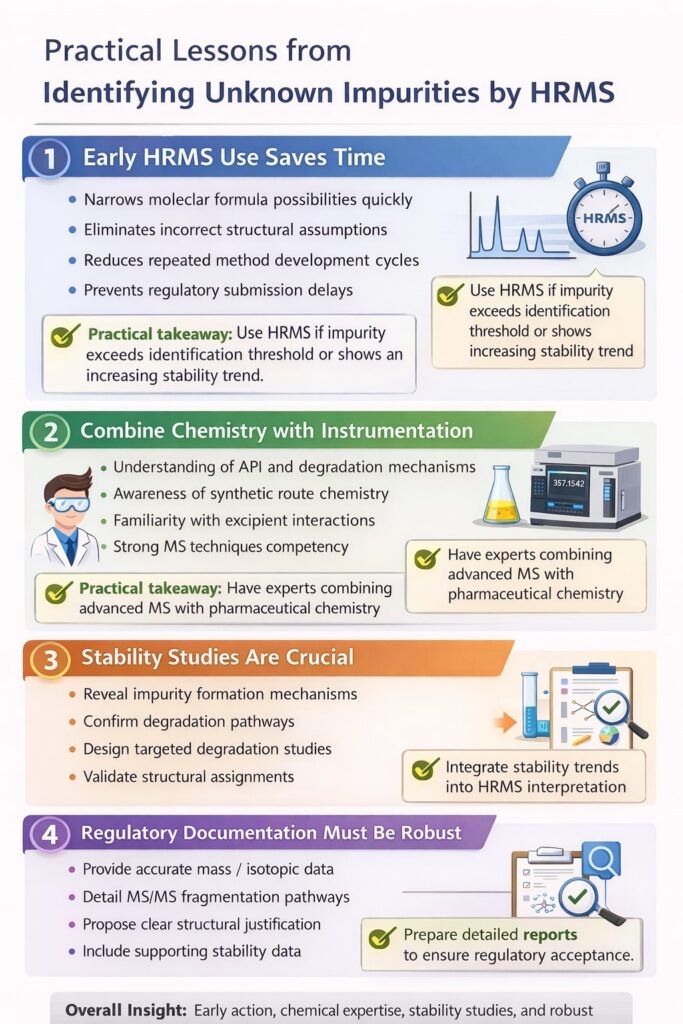
This case study demonstrates that successful Identifying Unknown Impurities by HRMS requires early action, chemical expertise, stability insight, and regulatory-ready documentation. Below are the key practical lessons pharmaceutical teams can apply immediately.
1. Early HRMS Use Saves Time
Escalating to HRMS early in the investigation significantly reduces delays and repeated analytical cycles.
Many teams initially rely on conventional LC-MS to conserve resources. However, when structural ambiguity persists, repeated testing can consume weeks without reaching a clear conclusion. Early adoption of Identifying Unknown Impurities by HRMS:
- Narrows molecular formula possibilities quickly
- Eliminates incorrect structural assumptions
- Reduces repeated method development cycles
- Accelerates root cause analysis
- Prevents regulatory submission delays
Practical takeaway: If an impurity exceeds identification thresholds or shows an increasing stability trend, move to HRMS immediately.
2. Combine Chemistry with Instrumentation
Identifying Unknown Impurities by HRMS is not just instrument-driven—it requires strong chemical reasoning.
High-resolution data provides exact mass and fragmentation patterns, but interpretation requires:
- Understanding of API functional groups
- Knowledge of degradation mechanisms
- Awareness of synthetic route chemistry
- Familiarity with excipient interactions
- Experience in oxidative, hydrolytic, and photolytic pathways
Instrumentation generates data; chemistry converts data into defensible conclusions.
Practical takeaway: Impurity identification should be handled by analysts who understand both advanced MS techniques and pharmaceutical chemistry.
3. Stability Studies Are Crucial
Trend analysis from stability studies often reveals the origin and mechanism of impurity formation.
In many cases, unknown impurities:
- Appear under accelerated conditions
- Increase with humidity exposure
- Form during oxidative stress
- Develop during long-term storage
By correlating impurity growth with stress conditions, investigators can:
- Propose mechanistic pathways
- Confirm degradation hypotheses
- Design targeted forced degradation studies
- Validate structural assignments
HRMS becomes even more powerful when interpreted alongside stability data.
Practical takeaway: Always integrate stability trends into the HRMS interpretation process to strengthen structural justification.
4. Regulatory Documentation Must Be Robust
Complete and defensible documentation is essential for regulatory acceptance of Identifying Unknown Impurities by HRMS findings.
Regulators expect clear scientific evidence, not assumptions. Documentation should include:
- Accurate mass measurements (ppm error reported)
- Isotopic pattern comparison
- MS/MS fragmentation maps
- Proposed structure with rationale
- Mechanistic formation pathway
- Supporting stress study data
- Chromatographic purity assessment
Well-prepared documentation:
- Reduces regulatory queries
- Strengthens ANDA/NDA submissions
- Minimizes back-and-forth correspondence
- Demonstrates scientific control
Practical takeaway: Treat impurity identification reports as regulatory documents, not just analytical summaries.
Overall Insight
This case study reinforces that Identifying Unknown Impurities by HRMS is a strategic scientific process—not just a laboratory test. When applied early, interpreted by experienced scientists, supported by stability data, and documented rigorously, HRMS transforms impurity investigations from reactive troubleshooting into controlled, defensible scientific analysis.
6: Our Scientific Approach at ResolveMass Laboratories Inc.
At ResolveMass Laboratories Inc., we follow a structured impurity investigation framework:
- Confirm chromatographic reproducibility
- Perform LC-HRMS analysis
- Conduct MS/MS fragmentation mapping
- Propose chemical structures
- Validate with stress studies or reference standards
- Provide regulatory-ready reports
Our analytical team combines:
- Advanced HRMS instrumentation
- Pharmaceutical impurity expertise
- Regulatory documentation experience
- Fast turnaround times
This ensures confidence when Identifying Unknown Impurities by HRMS for critical drug development projects.
Conclusion:
Identifying Unknown Impurities by HRMS is essential for ensuring pharmaceutical safety, regulatory compliance, and product quality. This case study demonstrates how high-resolution mass spectrometry, combined with chemical expertise and regulatory understanding, can quickly solve complex impurity challenges.
At ResolveMass Laboratories Inc., we help pharmaceutical companies move from uncertainty to clarity with science-backed, regulator-ready impurity identification services.
Frequently Asked Questions :
Identification typically follows a structured workflow:
-Confirm peak reproducibility (HPLC/UPLC with PDA).
-Assess peak purity.
-Obtain accurate mass using HRMS.
-Determine molecular formula (ppm error + isotopic pattern).
-Perform MS/MS fragmentation.
-Correlate with stability/stress studies.
-Confirm with reference standard or synthesis (if required).
In pharmaceutical development, Identifying Unknown Impurities by HRMS is the gold standard when impurities exceed ICH thresholds.
Mass spectrometry identifies compounds by:
-Measuring exact mass (m/z)
-Determining molecular formula
-Analyzing isotopic distribution
-Studying fragmentation patterns (MS/MS)
-Comparing against databases or reference standards
High-resolution systems allow structural hypothesis generation with high confidence.
High-Resolution Mass Spectrometry (HRMS) is used for:
-Molecular formula determination
-Impurity identification
-Degradation product characterization
-Metabolite identification
-Extractables & leachables analysis
-Forensic and environmental analysis
-Proteomics and biomarker discovery
In pharmaceuticals, HRMS is especially critical for regulatory impurity investigations.
In pharmaceutical analysis, common impurities include:
-Process-related impurities
-Degradation products (oxidation, hydrolysis, photolysis)
-Residual solvents
-Reagent by-products
-Isomeric impurities
-Packaging extractables/leachables
In MS instrumentation itself, background signals may arise from:
-Plasticizers
-Column bleed
-Solvent contaminants
-Adduct ions (Na⁺, K⁺)
The most reliable approach combines:
-Chromatography (separation)
-High-resolution MS (exact mass)
-MS/MS fragmentation
-Orthogonal techniques (NMR, IR if needed)
-Reference standard confirmation
For small molecules in pharma, LC-HRMS + MS/MS is usually the most efficient first-line strategy.
Mass spectrometry cannot directly detect:
-Species that cannot be ionized
-Extremely unstable molecules that decompose before detection
-Very low-level analytes below instrument sensitivity
-Some highly nonpolar gases without specialized ionization methods
Also, MS does not directly provide full 3D structure without supporting data.
The four fundamental stages are:
1. Ionization – Convert molecules to ions
2. Acceleration – Accelerate ions in an electric field
3. Separation – Separate ions by mass-to-charge ratio (m/z)
4. Detection – Measure ion abundance
-MS1: First stage mass scan measuring intact precursor ions.
-MS2 (MS/MS): Second stage where selected precursor ions are fragmented to generate product ions for structural elucidation.
MS2 provides structural information beyond molecular weight.
A systematic approach:
-Determine molecular weight (MS).
-Calculate molecular formula (HRMS).
-Analyze fragmentation (MS/MS).
-Compare with known databases/literature.
-Correlate with chemical knowledge and degradation pathways.
-Confirm with authentic standard if required.
Definitive identification usually requires converging multiple lines of evidence.
Key limitations include:
-Cannot always distinguish structural isomers without additional techniques.
-Requires ionization capability.
-Matrix effects can suppress ionization.
-Quantitation may require validated calibration.
-Expensive instrumentation and skilled interpretation required.
-May need complementary techniques (NMR, XRD).
Despite limitations, HRMS remains one of the most powerful tools for identifying unknown impurities in regulated pharmaceutical environments.
Reference
- Absolute Quantitation of Coeluting Impurities in Peptide Drugs Using High Resolution Mass Spectrometry: Glucagon a Case Study in Pharmaceutical Development.https://pubs.acs.org/doi/abs/10.1021/jasms.5c00105
- Rapid and Comprehensive Impurity Profiling of Synthetic Thyroxine by Ultrahigh-Performance Liquid Chromatography–High-Resolution Mass Spectrometry.https://pubs.acs.org/doi/abs/10.1021/ac303722j
- Masaaki Ubukata , Karl J. Jobst , Eric J. Reiner , Stephen E. Reichenbach , Qingping Tao , Jiliang Hang , Zhanpin Wu , A. John Dane , Robert B. Cody.Non-targeted analysis of electronics waste by comprehensive two-dimensional gas chromatography combined with high-resolution mass spectrometry: Using accurate mass information and mass defect analysis to explore the data.https://www.sciencedirect.com/science/article/abs/pii/S0021967315004604
- Advances in Ultra-High-Resolution Mass Spectrometry for Pharmaceutical Analysis.https://www.mdpi.com/1420-3049/28/5/2061
- Estelle Deschamps , Valentina Calabrese , Isabelle Schmitz , Marie Hubert-Roux , Denis Castagnos , Carlos Afonso. Advances in Ultra-High-Resolution Mass Spectrometry for Pharmaceutical Analysis.https://www.mdpi.com/1420-3049/28/5/2061
- Laura Backer , Helmut Buschmann , Martina Kinzig , Fritz Sörgel , Oliver Scherf-Clavel. Application of advanced high resolution mass spectrometric techniques for the analysis of losartan potassium regarding known and unknown impurities.https://www.sciencedirect.com/science/article/abs/pii/S0731708523007240

