Summary:
- Objective: Demonstrate a regulator-ready Impurity Control Strategy in CMC using a real-world NDA-style case study.
- Scope: End-to-end impurity lifecycle—from risk assessment and identification through justification, control, and lifecycle management.
- Regulatory Focus: Alignment with ICH Q3A/B, Q3C, M7, Q6A, and current FDA/EMA expectations for NDAs.
- Outcome: A defensible impurity strategy that withstands agency questions, supports specifications, and reduces approval risk.
- Key Takeaway: Successful NDA submissions hinge on data-backed impurity understanding, not generic controls.
Introduction
This case study explains how a complete Impurity Control Strategy in CMC was designed, justified, and clearly documented for an NDA submission. Instead of relying on high-level summaries or standard templates, the strategy was built using actual impurity data and detailed process knowledge. Strong focus was placed on how impurities were identified, assessed for risk, controlled within manufacturing, and explained to regulators. The example reflects real-world experience from late-stage development, commercial scale-up, and NDA preparation. It highlights the depth, clarity, and scientific thinking regulators expect when reviewing impurity sections.
Learn how specialized expertise can streamline your regulatory path: Explore our IND & CMC Case Studies for Drug Discovery
Case Context: NDA Program and Regulatory Expectations
The NDA involved a synthetic small-molecule API moving from late Phase II into commercial manufacturing, which increased regulatory focus on impurity control across the entire lifecycle.
Program Snapshot
| Attribute | Details |
|---|---|
| Dosage form | Immediate-release oral tablet |
| API source | Synthetic, multi-step process |
| Daily dose | 200 mg |
| Development stage | NDA submission |
| Regulatory markets | US (FDA), EU (EMA) |
At the NDA stage, regulators expect the Impurity Control Strategy in CMC to be complete, mature, and fully supported by data. Impurity profiles should be consistent and well understood, with clear links to the manufacturing process. Assumptions or evolving hypotheses that may be acceptable earlier in development are no longer sufficient. Each actual or potential impurity must be traceable to its source and managed using a clear scientific rationale.
Bridge the gap between development and commercialization: Discover our Comprehensive Drug Discovery CRO Chemistry Services
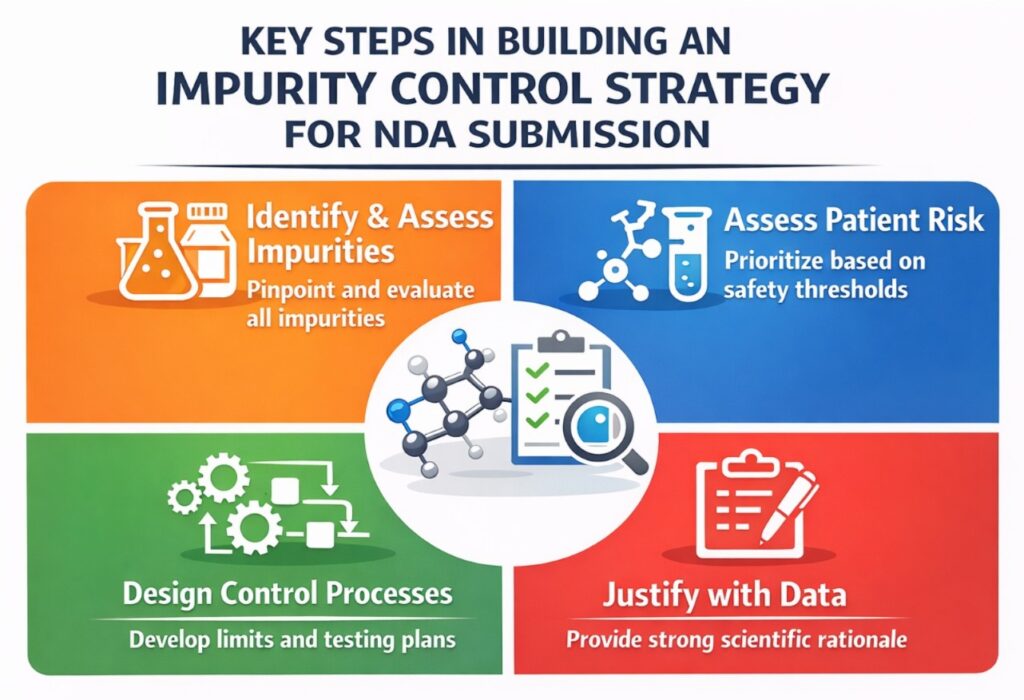
Impurity Risk Assessment Strategy in CMC (NDA-Driven)
A structured, risk-based approach was used to rank impurities based on patient safety impact, likelihood of formation, and ability to detect and control them.
Methodology Applied
- Detailed process mapping across all synthetic steps to identify where impurities could be introduced or formed
- Risk evaluation of reagents, solvents, catalysts, and intermediates used during manufacturing
- Forced degradation and stress studies to understand possible degradation pathways
- Application of toxicological thresholds aligned with ICH M7 and ICH Q3A/B
This systematic assessment became the backbone of the Impurity Control Strategy in CMC. It ensured that development efforts were focused on impurities that mattered most from both safety and regulatory perspectives.
Optimize your chemistry strategy for biotech success: Learn about Outsourced Chemistry Services for Biotech
Risk Categorization
- High-risk: Potential mutagenic impurities (PMIs) and impurities formed in late-stage synthesis
- Medium-risk: Known process-related impurities with proven purge capability
- Low-risk: Early-stage impurities shown to be effectively removed during downstream processing
Impurity Identification: What Was Actually Found and Why It Mattered
Twelve impurities were identified in total, and five required detailed discussion and justification in the NDA.
Identified Impurity Classes
- Process-related impurities (PRIs):
- Residual starting materials
- Reaction by-products and side products
- Degradation impurities:
- Oxidative degradants
- Hydrolytic degradants
- Residual solvents:
- Class 2 solvents present close to ICH Q3C limits
Analytical Approach Used
| Technique | Purpose |
|---|---|
| LC–HRMS | Structural elucidation |
| GC–MS | Detection of volatiles |
| NMR | Confirmation of unknowns |
| Stress testing | Generation of degradants |
Each impurity was either fully identified or scientifically justified based on known chemistry and degradation behavior. This level of characterization is a key requirement for an NDA-ready Impurity Control Strategy in CMC and helps reduce uncertainty during regulatory review.
Ensure your analytical methods are ready for regulatory scrutiny: Custom Analytical Method Development for Drug Discovery
Mutagenic Impurity Assessment and Justification
Two theoretical PMIs were assessed and shown to be controlled below the TTC using strong purge arguments and supporting data.
Regulatory Framework Applied
- ICH M7 (R2) classification and risk assessment
- In silico (QSAR) analysis to identify mutagenicity alerts
- Purge factor calculations across relevant process steps
Key Outcomes
- Routine testing for PMIs was not required due to strong purge evidence
- Justification was based on reaction chemistry, mass balance, and impurity spiking studies
- Acceptable intake limits were directly linked to the maximum daily dose
This science-based justification strengthened confidence in the Impurity Control Strategy in CMC and reduced follow-up questions from regulators.
Support your safety studies with precise chemical markers: Explore Isotopic Labeling Services for Drug Discovery
Control Strategy Design Across the Manufacturing Lifecycle
Several layers of control were implemented to ensure consistent and reliable impurity management.
Core Elements of the Impurity Control Strategy in CMC
- Process controls:
- Defined reaction stoichiometry
- Controlled temperature and pH ranges
- In-process controls (IPCs):
- Monitoring of critical steps where impurities may form
- Analytical controls:
- Stability-indicating analytical methods
- Specifications:
- Clearly defined impurity limits for API and drug product
Control Mapping Table
| Impurity Type | Control Point | Control Mechanism |
|---|---|---|
| Process impurity A | Step 3 reaction | IPC limit |
| Degradant D | Stability | Shelf-life spec |
| Residual solvent | Final API | Release testing |
This layered approach clearly demonstrated a robust Impurity Control Strategy in CMC, rather than reliance on final testing alone.
Accelerate your path to clinic with efficient chemistry support: CRO Chemistry Services Timelines for Drug Discovery
Setting and Justifying Impurity Specifications for NDA
Impurity limits were set using safety data and manufacturing capability, not default pharmacopeial values.
Justification Package Included
- Batch analysis data from at least ten commercial-scale batches
- Long-term and accelerated stability studies
- Toxicological qualification where required
Example Specification Rationale
- Impurity X set at a limit of 0.15% based on:
- No adverse toxicological findings
- Consistent batch results below 0.10%
- Demonstrated manufacturing control margin
Because the Impurity Control Strategy in CMC clearly linked safety and process performance, regulators accepted the proposed specifications.
Find cost-effective solutions for your medicinal chemistry needs: Cost-Effective Drug Discovery Chemistry Services
Handling Unknown and Unspecified Impurities
Unknown impurities were actively investigated instead of being ignored or postponed.
Actions Taken
- Reporting thresholds were lowered during development to detect trends early
- Structural identification was attempted for unknowns above 0.05%
- Remaining unknowns were conservatively controlled under unspecified impurity limits
This proactive approach reduced regulatory concern and strengthened the overall impurity narrative.
Leverage high-level expertise without the overhead: Learn about our Virtual Chemistry Department CRO
NDA Submission Strategy: How Impurity Data Was Presented
Impurity data was presented as one consistent story across CTD modules.
CTD Alignment
- Module 3.2.S: API impurity identification, justification, and control strategy
- Module 3.2.P: Clear linkage between API impurities and drug product degradants
- Module 2.3: High-level summary of the Impurity Control Strategy in CMC
Consistent presentation across modules helped reviewers quickly understand the strategy and rationale.
Access local expertise in North America: Drug Discovery CRO in Canada
Regulatory Review Outcomes and Lessons Learned
No major deficiency letters related to impurities were issued.
Key Success Factors
- Early ownership and assessment of impurity risks
- Strong analytical data and structural understanding
- Clear, transparent, and data-driven justifications
Practical Lessons
- Most late-stage impurity issues can be avoided with early planning
- Conservative, science-based limits build regulatory trust
- A strong Impurity Control Strategy in CMC can shorten NDA review timelines
Conclusion
This case study shows that an NDA-ready Impurity Control Strategy in CMC must be built on solid process understanding, reliable analytical data, and clear regulatory reasoning. Generic compliance statements are not enough at this stage of development. By anticipating reviewer expectations and presenting impurity data as a unified and logical strategy, sponsors can reduce approval risk and avoid post-approval commitments. Ultimately, a well-designed Impurity Control Strategy in CMC protects patients and supports long-term product success.
Contact ResolveMass Today – Contact Us
Frequently Asked Questions (FAQs)
For an NDA, regulators expect a clear and well-supported understanding of impurities. Any impurity above the reporting threshold should be structurally identified whenever possible. If full identification is not feasible, a strong scientific justification based on chemistry and analytical data is required.
Mutagenic impurity studies become necessary when there is a potential risk of DNA reactivity. This usually occurs when certain reagents, intermediates, or degradation pathways raise structural alerts under ICH M7. In such cases, risk assessment and appropriate control strategies must be applied.
Yes, purge arguments can replace routine testing if they are scientifically sound. Regulators accept purge-based justifications when supported by reaction chemistry, experimental data, and a clear understanding of impurity removal across process steps.
In most cases, data from six to ten commercial-scale or representative batches is sufficient. These batches should demonstrate consistent impurity control and process capability. The data must clearly support the proposed limits in the specification.
Stability data shows how impurities and degradants behave over the product’s shelf life. It helps confirm that impurity levels remain within acceptable limits under long-term and accelerated conditions. This data is critical for setting and justifying shelf-life specifications.
Regulatory questions usually arise when impurity data is inconsistent or poorly justified. Gaps in rationale, unexplained variability, or missing links to the manufacturing process often raise concerns. Clear, data-driven explanations help avoid such issues.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas