Summary: Key Insights at a Glance
- Outsourcing IND CMC Services can accelerate timelines, reduce risk, and ensure compliance with evolving FDA and ICH standards.
- The right timing for outsourcing depends on development phase, internal capabilities, and regulatory complexity.
- Strategic outsourcing can fill technical gaps in analytical development, formulation, and process validation.
- Data integrity, traceability, and regulatory documentation are non-negotiable success factors in outsourced CMC programs.
- Working with specialized partners ensures end-to-end coordination between preclinical, manufacturing, and regulatory teams.
- Outsourcing enables early-stage biotech firms to compete with larger organizations without infrastructure burden.
- The key is selecting a CMC service provider with proven experience, transparent quality systems, and strong communication frameworks.
Introduction: Why Outsourcing IND CMC Services Is a Strategic Move
Outsourcing IND CMC Services is no longer only about saving costs—it has become a strategic way to accelerate development while maintaining regulatory confidence. As IND submissions grow more complex, the CMC section requires a high level of scientific detail, data accuracy, and documentation control. Many organizations find it difficult for internal teams alone to meet these growing demands efficiently. Outsourcing provides access to professionals who understand regulatory scrutiny in depth.
For companies preparing an Investigational New Drug (IND) application, the Chemistry, Manufacturing, and Controls section is often the most time-consuming and resource-heavy part. It involves extensive analytical data, clearly defined manufacturing processes, and strong control strategies. Any missing or unclear information can result in FDA information requests or even clinical holds. External CMC experts help ensure the submission is complete and consistent from the beginning.
Pharmaceutical and biotech innovators increasingly rely on specialized CMC partners with strong expertise in formulation science, analytical development, and GMP manufacturing. These partners have real-world experience responding to regulatory feedback. Their insights help sponsors anticipate reviewer concerns before submission. This proactive approach significantly strengthens IND readiness.
This collaborative model reduces the risk of regulatory delays, prevents data gaps, and improves readiness for key clinical milestones. Sponsors benefit from running CMC activities in parallel with preclinical and clinical planning. Speed does not compromise quality when experienced partners are involved. Instead, both development pace and regulatory confidence improve.
Looking for comprehensive support? Explore our full suite of Chemistry, Manufacturing, and Controls (CMC) Services to ensure your submission is complete and consistent.
1. The Critical Timing: When to Outsource IND CMC Services
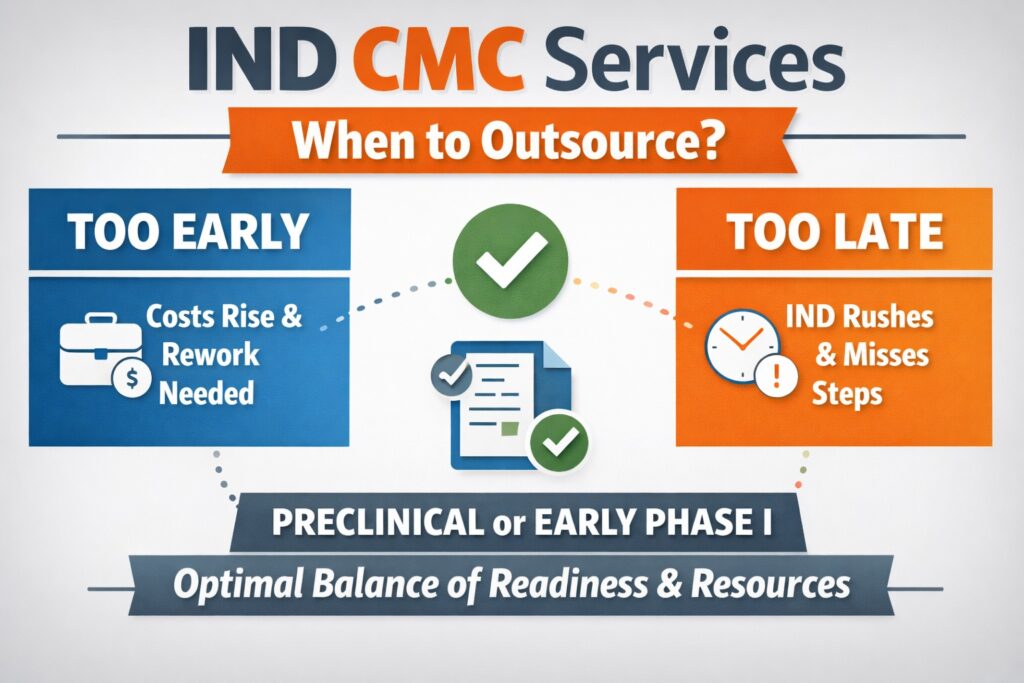
The decision of when to outsource IND CMC Services can directly impact IND success. Engaging too early may create inefficiencies if the drug candidate is not well defined. Waiting too long often leads to rushed development and incomplete documentation. The best timing is when technical clarity starts to take shape.
In most cases, outsourcing is most effective during late preclinical development or early Phase I planning. At this stage, formulation feasibility, analytical methods, and manufacturing strategies must be finalized. External partners ensure these activities are performed under regulatory-ready conditions. This timing supports smoother IND preparation and submission.
Outsourcing too early can increase costs and cause rework, while delaying outsourcing can slow regulatory timelines. Sponsors must carefully balance readiness with resource use. A phased outsourcing strategy often works best. Early engagement focused on critical deliverables helps reduce downstream risk.
Ideal Timing for Outsourcing IND CMC Services
| Development Stage | Key CMC Focus | When to Outsource | Rationale |
|---|---|---|---|
| Discovery | Candidate selection | Optional | Internal work often sufficient |
| Preclinical | Formulation feasibility, analytical method development | ✅ Recommended | Builds strong IND data |
| Phase I | Process development, stability studies | ✅ Critical | Supports early clinical manufacturing |
| Phase II | Process optimization, tech transfer | Partial | Hybrid model works well |
| Phase III | Scale-up, validation | Often in-house or CMO | Higher regulatory rigor |
In summary, outsourcing delivers the most value once a lead compound is confirmed and compliant, scalable manufacturing data are required. Regulatory expectations increase sharply at this stage. External expertise ensures alignment with IND requirements and supports timely review.
Need to bridge the gap between discovery and the clinic? Learn how our Drug Discovery Services set the foundation for a successful IND.
2. Why Outsourcing CMC Services Enhances IND Readiness
The main reason to outsource IND CMC Services is to ensure reliable data and regulatory compliance across chemistry, formulation, and manufacturing controls. Each area demands specialized expertise and validated systems. Even small errors can impact the entire IND package. Outsourcing lowers this risk through standardized processes.
Leading CMC partners use cross-functional teams that include regulatory scientists, engineers, and quality experts. These teams understand FDA expectations as well as international requirements from EMA and Health Canada. Their integrated approach improves consistency across all datasets. This alignment strengthens submission credibility.
Well-prepared documentation reduces the chance of IND hold letters and follow-up questions. Clear justifications and validated methods help regulators review submissions more efficiently. This directly supports faster development timelines.
Additional benefits include:
- Regulatory Predictability: Documentation aligned with FDA eCTD Module 3 and current guidance
- Technical Continuity: Smooth transition from early development to GMP manufacturing
- Cost Efficiency: Avoids heavy investment in internal analytical infrastructure
- Risk Mitigation: Reduces errors in validation, stability studies, and release testing
By using external expertise, sponsors ensure that every assay, process step, and batch record follows current CMC guidance. This consistency builds regulatory trust and supports future lifecycle management activities.
Ready to move from Phase I toward market approval? Discover our specialized CMC Services for IND and NDA applications.
3. Strategic Drivers for Outsourcing IND CMC Services
Organizations outsource IND CMC Services for reasons that go beyond staffing limitations. As development programs become more complex, internal teams may lack niche capabilities. Outsourcing fills these gaps without long-term commitments and adds flexibility to development planning.
a. Access to Specialized Expertise
Advanced drug modalities such as biologics, mRNA therapies, and antibody-drug conjugates require specialized analytical and manufacturing knowledge. These products involve complex characterization and control strategies. Outsourcing provides immediate access to experts with proven experience. This improves data strength and regulatory acceptance.
Protect your timeline from mutagenic impurity concerns with our expert Nitrosamine Analysis.c
b. Accelerated Timelines
Dedicated CMC partners work in parallel with sponsor teams, shortening overall development timelines. Activities like method validation and stability testing can run simultaneously. This parallel execution is often difficult to achieve internally.
c. Scalable Manufacturing Solutions
Many early-stage companies lack GMP manufacturing facilities. Outsourcing provides access to GMP-compliant production without large capital investment. Sponsors can scale production as needed while avoiding long-term infrastructure costs.
d. Regulatory Navigation
Experienced partners guide sponsors through IND Module 3 preparation and alignment with FDA and ICH Q8–Q10 guidelines. This guidance reduces uncertainty and helps plan for future regulatory expectations.
e. Focus on Core Science
By outsourcing CMC execution, internal teams can focus on discovery, translational research, and clinical strategy. This improves productivity and keeps scientists focused on innovation rather than operational complexity.
4. Balancing Control and Flexibility in IND CMC Services Outsourcing
One challenge of outsourcing IND CMC Services is maintaining quality oversight while allowing partners to work efficiently. Sponsors remain responsible for regulatory submissions, but partners need flexibility to deliver technical work. Achieving this balance is essential.
A co-managed model is widely considered best practice. Sponsors retain regulatory ownership while partners execute defined tasks. Roles and responsibilities are clearly documented to support accountability.
Clear quality agreements, data-sharing procedures, and audit trails promote transparency. These measures set expectations early and reduce misunderstandings. Transparency is critical for regulatory confidence.
Key elements include:
- Joint governance committees
- Alignment with the sponsor’s quality management system
- Defined method transfer procedures
- Real-time data access through validated systems
A strong CMC partner becomes part of the sponsor’s quality ecosystem, not just a service provider.
Ensure your testing methods meet rigorous regulatory standards with our Analytical Method Development and Validation Service.
5. Cost-Efficiency Without Compromising Quality
Outsourcing IND CMC Services is about smart cost management, not cutting corners. Internal development involves high fixed costs, including equipment, staffing, and facility maintenance. Outsourcing converts these into flexible, milestone-based expenses.
Choosing the lowest-cost provider can be risky. Weak quality systems may compromise data integrity and lead to regulatory delays. Quality should always come first.
Indicators of a reliable and cost-efficient provider include:
- Transparent pricing models
- Validated analytical and stability systems
- Proven IND submission experience
- Secure data management practices
True value lies in achieving regulatory reliability with efficient investment. A strong IND submission avoids costly delays later.
Understand your molecule’s stability profile early. Explore our Forced Degradation Studies to prevent costly delays later in development.
6. Data Integrity and Compliance at the Core of IND CMC Services
Regulatory agencies place high importance on data integrity. Outsourced partners must follow ALCOA+ principles to ensure data is accurate, traceable, and reliable. Any gaps can weaken the IND.
Validated electronic systems ensure proper traceability and inspection readiness. All records must be complete, attributable, and contemporaneous.
Key compliance checkpoints include:
- Complete batch records and deviation tracking
- Robust CAPA systems
- Inspection readiness aligned with FDA, EMA, and Health Canada
Strong data governance protects both the IND submission and future inspections.
Ensure product safety and container compatibility with our Extractables and Leachables Testing.
7. Integrating IND CMC Services with Regulatory Strategy
Outsourcing is most effective when CMC activities are fully aligned with regulatory strategy. Early decisions affect future filings and commercial plans. Alignment reduces rework and regulatory risk.
CMC and regulatory teams must collaborate on formulation choices, stability programs, and control strategies. These decisions directly influence IND acceptance.
Additional integration points include:
- Supporting comparability and tech transfer
- Aligning control strategies with long-term goals
- Maintaining flexibility for post-IND updates
Experienced partners bridge technical execution with regulatory planning.
Confused about the transition from clinical to commercial requirements? Read our guide on IND vs NDA CMC Requirements to stay ahead.
8. Choosing the Right Partner for IND CMC Services
Selecting the right provider for IND CMC Services is critical. Experience, quality systems, and communication matter as much as capacity and cost.
| Criteria | Why It Matters |
|---|---|
| IND submission track record | Shows regulatory experience |
| Analytical and formulation capability | Ensures full CMC coverage |
| Quality systems | Confirms cGMP and GLP compliance |
| Communication transparency | Enables fast issue resolution |
| Regulatory support | Aligns execution with submissions |
The ideal partner offers strong science, documentation discipline, and a collaborative mindset.
Conclusion: Making IND CMC Outsourcing a Competitive Advantage
In today’s fast-paced development environment, outsourcing IND CMC Services has become a competitive advantage. When done correctly, it improves speed, quality, and regulatory confidence. Timing, partner selection, and oversight define success.
By combining technical excellence with compliance and clear communication, sponsors can achieve faster IND clearance and sustainable development efficiency.
ResolveMass Laboratories Inc. follows this model by blending scientific expertise with regulatory insight. Their approach supports complete, compliant, and inspection-ready IND submissions.
👉 Contact ResolveMass Laboratories
FAQs: Outsourcing IND CMC Services
A biotech company should consider outsourcing IND CMC Services during late preclinical development or early Phase I planning. This is usually the stage when analytical methods, formulation, and manufacturing strategies must meet regulatory standards. Outsourcing at this point helps avoid rushed work and last-minute gaps. It also supports a smoother and more confident IND submission.
No, outsourcing does not change regulatory ownership of the IND. The sponsor always remains fully responsible for the submission and all regulatory interactions. External partners only support technical execution and documentation. Regulatory accountability stays with the sponsor at all times.
Outsourcing can significantly shorten IND timelines when planned correctly. External partners can run CMC activities in parallel with internal preclinical and clinical planning. This reduces waiting time and avoids workflow bottlenecks. As a result, sponsors often see faster and more predictable submissions.
The main risks include data inconsistencies, communication gaps, or audit findings if the provider lacks a strong quality system. These risks can be minimized by choosing an experienced partner. Clear quality agreements and regular oversight are essential. Proper governance ensures data reliability and compliance.
Data security is maintained through validated electronic systems and controlled access protocols. Reputable partners use secure data transfer methods and audit trails. All data is tracked, traceable, and protected from unauthorized access. This ensures regulatory and confidentiality requirements are met.
Reference
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196