🔍 Summary of Key Points
- CMC strategy is the critical foundation of a successful First-in-Human (FIH) IND application.
- Early phase programs must balance speed, risk mitigation, and regulatory expectations.
- A robust IND CMC Strategy accelerates timelines, controls cost, and ensures regulatory compliance.
- Preclinical and clinical supply chain planning is inseparable from CMC execution.
- FDA expectations for FIH INDs differ from late-phase filings—right-sizing data is key.
- Proactive risk assessments, phase-appropriate controls, and scalable processes define success.
- Critical documentation includes drug substance (DS), drug product (DP), analytical methods, and stability protocols.
- Strategic outsourcing, technology transfer planning, and QbD principles enable scalable manufacturing.
- Failures in CMC are a top reason for IND clinical holds—strategic CMC planning avoids them.
🧪 Introduction: Why IND CMC Strategy Drives FIH IND Success
A well-prepared IND CMC Strategy is critical to the success of First-in-Human (FIH) clinical trials. It gives regulators confidence that the drug substance and product are manufactured, tested, and controlled according to FDA guidelines. At this early stage, the focus is not on large-scale production, but rather on ensuring patient safety and consistent product quality.
A strong CMC approach reduces uncertainty by clearly explaining the manufacturing process, quality tests, and product stability plans. Without a clear strategy, even a promising drug can face delays or clinical holds due to regulatory concerns.
Early-stage companies often struggle with balancing quick entry into clinical trials and regulatory compliance. Investing too much can waste time and money, while under-preparing increases the risk of rejection. A thoughtful IND CMC Strategy helps avoid these pitfalls and supports a smoother journey into later clinical phases.
Expert Resource: Explore our comprehensive Chemistry, Manufacturing, and Controls (CMC) Services to streamline your path to clinical trials.
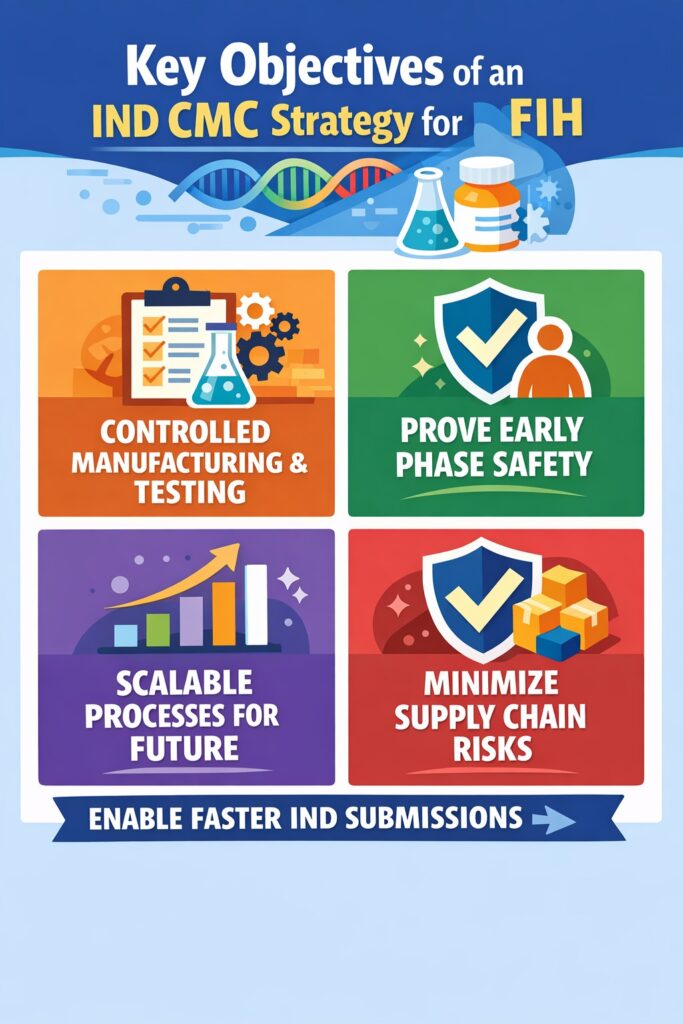
🧭 Strategic Objectives of an IND CMC Strategy for FIH
The goal of an IND CMC Strategy at the First-in-Human stage is to build a compliant and flexible framework that enables clinical progress. It should show that the manufacturing and testing processes are controlled, without making them too complicated for this phase.
Regulatory authorities expect clear reasoning for all decisions, backed by risk-based justifications. A good strategy also allows for an easier transition into Phase 2 without the need to redo previous work.
Key Objectives:
- Show clear control over how the drug is made and tested.
- Provide enough data to prove the product is safe for early human trials.
- Design processes that can be scaled later without major changes.
- Reduce risks in the supply chain that might cause clinical delays.
- Enable faster, compliant IND submissions with fewer regulatory problems.
📋 Core Components of an IND CMC Strategy
A well-structured IND CMC Strategy should cover all the major technical components needed to meet FDA standards. These elements should be prepared in a way that fits the early clinical phase, while still being suitable for submission in a Common Technical Document (CTD) format.
Planning ahead ensures that current data can also support future regulatory filings. A clear and coordinated approach helps reduce inconsistencies and follow-up questions from reviewers.
| Component | Focus Area |
|---|---|
| Drug Substance (DS) | Route of synthesis, impurities, specifications, control strategy |
| Drug Product (DP) | Formulation, fill-finish process, container-closure, stability |
| Analytical Methods | Phase-appropriate validation, robustness, trending |
| Stability | Real-time and accelerated studies, bracketing/matrixing where applicable |
| Process Development | Early QbD principles, scale-up potential, risk assessments |
| Regulatory Documents | Module 3 CTD-ready data and formatting |
For teams balancing multiple development stages, understanding the specific IND CMC requirements is vital for a successful filing.
⚙️ Phase-Appropriate Manufacturing Controls
In the First-in-Human stage, the FDA does not expect full commercial manufacturing controls. However, the agency does require proof that the product can be made consistently and safely. Manufacturing controls must be scientifically justified and matched to the intended clinical use.
Overcomplicating controls at this phase can add unnecessary workload without improving product quality. Instead, fit-for-purpose controls should include:
- Simple but effective in-process controls tied to product quality.
- Clearly defined critical quality attributes (CQAs) focused on patient safety.
- A documented plan for handling changes after IND submission.
- Risk-based reasoning for process variability.
This approach keeps the manufacturing process flexible while still ensuring it can be validated later.
🔬 Analytical Method Readiness in an IND CMC Strategy
Analytical methods are one of the most closely reviewed parts of an IND CMC Strategy. Even in early stages, these methods must show they can reliably measure product quality.
Incomplete or unclear analytical strategies often lead to regulatory questions or holds. A solid analytical plan builds confidence in the entire CMC package.
At this stage, the following are essential:
- Methods should be validated for early-phase use.
- Identity, purity, and potency tests must be well-qualified.
- Plans for tracking trends and comparability should be created early.
- Methods must be stability-indicating and scientifically justified.
Setting up strong analytical methods early prevents future setbacks and delays.
Technical Support: Need assistance with your testing protocols? Learn about our Analytical Method Development and Validation Services.
⏱️ Stability Strategy Tailored for FIH Development
A clear stability plan is necessary to show the product will remain safe and effective during the trial period. For FIH IND submissions, six months of stability data under ICH conditions is usually enough. However, this is only the starting point.
The strategy should also include plans for ongoing monitoring throughout the clinical study. Best practices include:
- Real-time stability testing on clinical batches.
- Use of representative batches with strong scientific support.
- Matrixing or bracketing approaches to reduce testing where appropriate.
- A clear explanation of how shelf-life was determined.
A proactive stability plan ensures there are no delays in trial material supply.
Beyond standard testing, regulators increasingly look for specialized safety data, such as Nitrosamine Analysis
and Forced Degradation Studies, to ensure long-term product integrity.
🧰 Technology Transfer and Outsourcing Within an IND CMC Strategy
Many early-stage programs rely on contract development and manufacturing organizations (CDMOs) to make their products. A strong IND CMC Strategy must clearly define how these partnerships are managed.
Sponsors are still fully responsible for product quality, even when outsourcing. To ensure success:
- Use detailed checklists for technology transfer to CDMOs.
- Evaluate CDMO capabilities for both current and future needs.
- Reduce risks by qualifying raw materials and aligning batch records.
- Verify data through parallel testing when necessary.
Proper oversight ensures outsourcing helps—not hurts—the development process.
Partner with Experts: Discover how our specialized CMC CRO Services can provide the oversight needed for high-quality data generation.
📈 Designing for Scalability and Commercialization
While early clinical success is the immediate goal, decisions made now will affect future phases and commercial readiness. Poorly designed processes may need to be redone later, costing time and money.
Applying forward-thinking design early on helps avoid these issues. Consider the following:
- Use platform manufacturing processes where possible.
- Plan early comparability studies to support future changes.
- Begin introducing quality by design (QbD) principles.
- Identify critical process parameters (CPPs) and learn from early results.
A scalable IND CMC Strategy builds a strong foundation for Phase 2 and commercialization.
As you move from discovery toward the clinic, integrating Drug Discovery Services with CMC planning ensures that your lead candidates are actually “manufacturable” at scale.
⚠️ Avoiding Common IND CMC Pitfalls
Many IND clinical holds result from preventable CMC mistakes. Common issues include:
- Incomplete or invalidated analytical methods.
- Missing impurity qualifications.
- Specifications that are too strict or not justified.
- Not enough detail in manufacturing steps.
- Poor documentation overall.
To avoid these problems:
- Conduct a formal CMC gap assessment before submission.
- Use pre-IND meetings with the FDA to clarify expectations.
- Justify any flexibility with risk-based reasoning.
- Keep all teams aligned—CMC, regulatory, and clinical.
🧑⚕️ Regulatory Considerations for IND CMC Submissions
Regulatory planning should be included from the very beginning. An IND CMC Strategy that addresses likely reviewer concerns can speed up approvals and reduce the number of follow-up questions.
Consistency and clarity across all CTD sections are critical. The FDA often focuses on:
- Drug substance and product specs aligned with safety requirements.
- Clearly listed manufacturing sites and batch records.
- Proof that clinical supply chains are ready.
- Well-organized Module 3 sections with supporting documents.
A strong submission is proactive, organized, and easy to review.
📌 IND CMC Strategy: Best Practices Checklist
| Best Practice | Benefit |
|---|---|
| Early risk identification | Reduces chances of IND holds |
| Phase-appropriate documentation | Helps meet FDA requirements |
| Outsourcing with oversight | Ensures product quality remains consistent |
| Right-sizing specifications | Maintains balance between control and flexibility |
| Integrated CMC-regulatory planning | Speeds up IND submission and approval |
| Data integrity and traceability | Builds regulatory trust and prevents extra work |
To avoid these, it is crucial to understand the nuances of IND vs. NDA CMC requirements to ensure your current data supports future transitions.
🏁 Conclusion: A Strong IND CMC Strategy Sets the Trajectory for Clinical Success
Investing time and resources into a strong, scalable IND CMC Strategy pays off throughout the drug development process. Early planning reduces delays, improves communication with regulators, and avoids costly mistakes down the road.
For First-in-Human trials, CMC readiness isn’t optional—it’s essential for patient safety and trial success. ResolveMass Laboratories brings deep industry knowledge and regulatory experience to help develop CMC strategies that move your program forward with confidence.
Our team emphasizes scientific accuracy, regulatory alignment, and long-term scalability to help you succeed at every stage of development.
📞 Need CMC Expertise for Your IND Filing?
Let our experienced team help you build an IND CMC Strategy tailored to your molecule’s unique needs and regulatory path.
FAQs on IND CMC Strategy
The main goal of an IND CMC Strategy is to demonstrate that the drug substance and drug product are consistently manufactured and controlled for safe First‑in‑Human use. It ensures regulatory compliance while remaining flexible for early development. This strategy helps regulators gain confidence in product quality and patient safety. It also prepares the program for smooth progression into later phases.
Full validation is not required during the FIH stage, but analytical methods must be fit for purpose. They should reliably assess identity, purity, and potency of the product. Phase‑appropriate validation helps demonstrate method suitability. Poorly developed methods can raise regulatory concerns and delay approval.
CMC‑related clinical holds often result from incomplete analytical validation, missing impurity data, or weak manufacturing controls. Unclear specifications and poor documentation are also common issues. These gaps can create safety or quality concerns for regulators. Early planning helps avoid these preventable delays.
A full QbD approach is not required for First‑in‑Human IND submissions. However, applying basic QbD principles early can be very beneficial. It helps identify risks, improve process understanding, and support future scalability. Early QbD thinking reduces rework in later phases.
An IND CMC Strategy helps biotech startups align early development with regulatory expectations. It reduces the risk of costly delays and unexpected clinical holds. A strong strategy also supports scalability and investor confidence. Ultimately, it helps startups move efficiently from discovery to the clinic.
Reference
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/
- Patel, D. H., Kumar, B. J., & Patel, A. A. (2017). Preparation and review of chemistry, manufacturing and control (CMC) sections of CTD dossier for marketing authorization. International Journal of Drug Regulatory Affairs, 5(2), 1–12. https://www.ijdra.com/index.php/journal/article/view/196
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas