Summary: Key Takeaways on IND vs NDA CMC Requirements
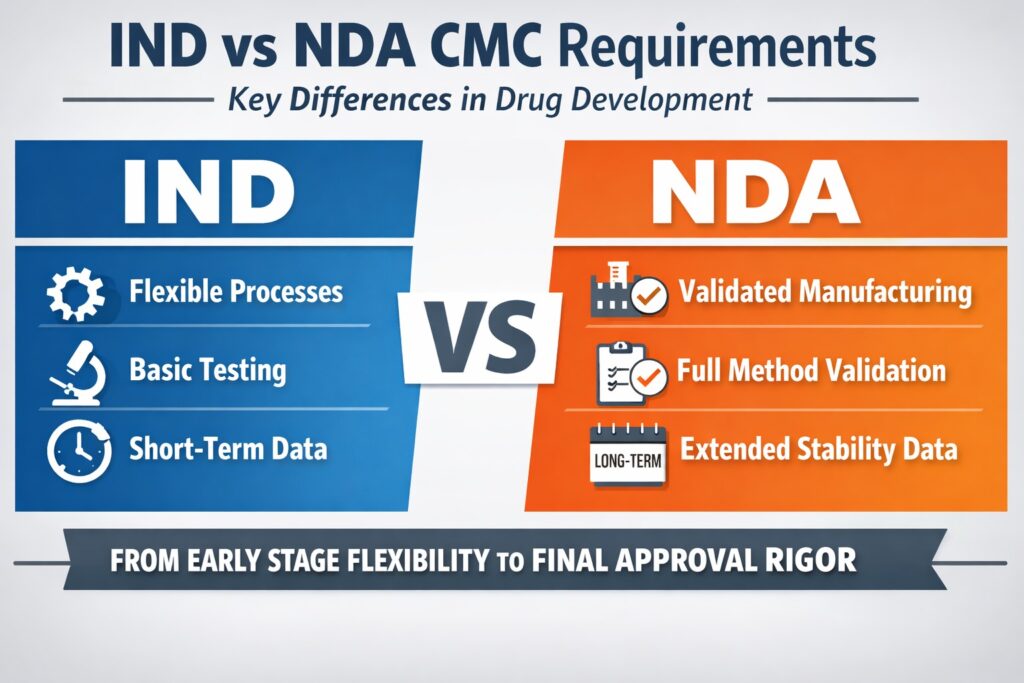
- CMC requirements evolve significantly from IND (Investigational New Drug) to NDA (New Drug Application) stage.
- The IND CMC focus is on safety and sufficient control to support human clinical trials.
- NDA CMC data demands full validation, stability, and lifecycle controls to support product approval and commercialization.
- Key changes occur in analytical validation, process control, formulation development, stability, and regulatory expectations.
- Sponsors must proactively adapt CMC strategies across development phases to avoid late-phase delays.
- This article breaks down each CMC shift with clear examples and a strategic lens for high regulatory success.
Introduction: Why IND vs NDA CMC Requirements Matter
Understanding the IND vs NDA CMC Requirements is critical for pharmaceutical and biotech companies preparing a product for market. The expectations grow as a drug progresses from clinical trials to final approval, and early-stage decisions can have lasting consequences.
If teams fail to align IND flexibility with NDA rigor, they may face delays, costly repeat studies, or reformulations. Anticipating what’s needed at the NDA stage—rather than reacting late—can improve efficiency and regulatory outcomes.
Need expert guidance? Explore our Chemistry, Manufacturing, and Controls (CMC) Services to streamline your path to approval.
This guide breaks down how CMC requirements change between IND and NDA, helping sponsors plan smarter, reduce risks, and stay compliant with FDA expectations.
CMC Requirements Comparison: IND vs NDA
IND vs NDA CMC Requirements: Key Differences at a Glance
| CMC Aspect | IND Requirements | NDA Requirements |
|---|---|---|
| Manufacturing Process | Defined at a high level; changes allowed | Fully validated and locked down |
| Process Validation | Not required; limited proof-of-concept data | Full process validation with PPQ data required |
| Analytical Methods | Can be qualified; limited validation acceptable | Fully validated per ICH Q2 guidelines |
| Formulation | Prototype formulation allowed | Final formulation must be justified and optimized |
| Stability Data | Minimum 3-month accelerated data | Long-term stability under ICH conditions; shelf-life justification |
| Specifications | May evolve during development | Must be final and justified; tight control over release specs |
| Site Qualification | Not necessary | All manufacturing and testing sites must be GMP-compliant and registered |
| Control Strategy | Preliminary, evolving | Comprehensive, lifecycle-ready strategy |
| Container Closure System | Basic info sufficient | Full compatibility, integrity, and safety data required |
This table shows how regulatory expectations become more stringent as a product moves toward market approval. Planning for these changes early avoids surprises during the NDA review process.
This table shows how regulatory expectations become more stringent as a product moves toward market approval. Planning for these changes early avoids surprises during the NDA review process. For a deeper dive into the documentation needed for each stage, see our specialized CMC Services for IND and NDA.
Process Development and Validation in IND vs NDA CMC Requirements
Process Control and Validation – IND vs NDA
At the IND stage, manufacturing processes are typically in development. Flexibility is allowed to support exploratory work, scale changes, and clinical trial material production.
However, NDA submissions must reflect a validated, fixed process ready for commercial use. Regulators want to see proof that the process consistently meets all required quality standards.
Accelerate your early-stage development with our comprehensive Drug Discovery Services.
IND Highlights:
- Manufacturing is still flexible and evolving
- No full-scale validation required
- Focus is on short-term production and safety
NDA Highlights:
- Commercial process is locked and validated
- PPQ (Process Performance Qualification) data mandatory
- Full demonstration of process control and reproducibility
Tip: Start process validation planning by Phase 2 to avoid rushed timelines at the NDA stage.
Analytical Method Development in IND vs NDA CMC Requirements
Method Qualification vs Full Validation
In early development, analytical methods only need to be suitable for characterizing safety and performance. Formal validation is not required at the IND stage.
NDA submissions must include fully validated methods per ICH Q2(R1) guidelines. These ensure data is reliable for product release, stability, and long-term quality monitoring.
Ensure your data meets regulatory rigor with our Analytical Method Development and Validation Service.
IND Stage:
- Methods can be qualified but not validated
- Acceptable to adjust methods during early trials
- Some parameters like specificity may be incomplete
NDA Stage:
- All methods must be validated (accuracy, precision, linearity, etc.)
- Required for release, stability, and control strategy
- Supports data integrity and regulatory confidence
Tip: Begin method validation activities by the end of Phase 2b.
Stability Data in IND vs NDA CMC Requirements
IND vs NDA Stability Data Requirements
For IND filings, stability data is minimal and only needs to cover the clinical trial period. Typically, this includes three months of accelerated or real-time data.
By the NDA stage, stability studies must justify product shelf life and support labeling claims. This requires long-term and accelerated data under ICH Q1A(R2) guidelines.
Prepare for long-term stability success with Forced Degradation Studies to identify potential impurities early.
IND Stage:
- Short-term data for clinical trial duration
- Limited justification for storage conditions
NDA Stage:
- 6–12 months long-term and 6 months accelerated data
- Full justification of shelf life and storage
- Supports product labeling and expiration dates
Tip: Align early stability studies with intended commercial formulation.
Formulation Development: IND Flexibility vs NDA Finalization
IND vs NDA CMC Requirements for Formulation
During IND, it’s acceptable to use prototype formulations to explore excipients and enhance bioavailability. This flexibility enables learning during early trials.
NDA formulations must be final, justified, and supported by performance and stability data. Post-IND changes may require bridging studies or bioequivalence data.
Mitigate risks by conducting Extractables and Leachables Testing to ensure container closure compatibility.
IND Phase:
- Exploratory formulation changes allowed
- No need to finalize excipient choices
NDA Phase:
- Formulation must be fixed and justified
- Any change must be supported with data
- Bioequivalence testing often required
Specifications: IND vs NDA CMC Strategy Shift
Finalizing Specifications from IND to NDA
Specifications start as broad guidelines during the IND phase, evolving as product and process knowledge grow. Regulators allow this flexibility to accommodate learning.
At the NDA stage, specifications must be tightly defined and supported with solid data. They are legally binding and aligned with the control strategy.
Stay compliant with the latest safety standards using our Nitrosamine Analysis services.
IND Specifications:
- Preliminary and subject to change
- Linked to ongoing development
NDA Specifications:
- Tight, data-backed limits
- Required for release and stability
- Integrated into lifecycle quality strategy
Regulatory Review Focus: IND vs NDA
FDA Review Priorities Across Development Stages
IND reviews focus on patient safety and whether the product is safe to test in humans. The goal is to ensure early-stage manufacturing supports clinical trials.
NDA reviews look at the big picture—does the drug consistently meet quality standards? Is the benefit-risk profile acceptable for approval?
IND Focus:
- Human safety
- Readiness for clinical testing
- Controls for clinical trial material
NDA Focus:
- Manufacturing quality and consistency
- Robust data supporting efficacy and safety
- Lifecycle management and risk planning
Expect a more detailed review during NDA, especially regarding process validation, comparability, and post-approval changes.
Site Compliance in IND vs NDA CMC Requirements
IND vs NDA Facility Readiness
Facilities used in the IND stage may not meet full commercial Good Manufacturing Practice (GMP) standards. They are often pilot-scale and not subject to pre-approval inspections.
For NDA, all sites involved in manufacturing, testing, and packaging must be GMP-compliant, registered, and ready for inspection by authorities like the FDA.
IND Stage:
- Pilot or non-commercial sites allowed
- No pre-approval inspections
NDA Stage:
- Sites must be GMP-certified
- Must pass pre-approval inspections
- Compliance issues can delay approval
Conclusion: Plan Early for IND vs NDA CMC Requirements
The difference between IND vs NDA CMC Requirements is not just about adding more data—it’s about establishing robust systems that ensure quality, consistency, and control.
By planning early, especially during Phase 2, sponsors can avoid costly delays and rework. Proactive CMC planning improves regulatory outcomes and supports successful commercialization.
ResolveMass Laboratories Inc. helps pharmaceutical and biotech companies bridge the CMC gap between IND and NDA through expert guidance, validation services, and regulatory support.
➡ Contact ResolveMass CMC Experts
FAQs on IND vs NDA CMC Requirements
The CMC requirements for an IND submission focus on basic safety controls and readiness to begin human trials. In contrast, the NDA stage demands validated processes, finalized specifications, and full control strategies to ensure consistent product quality throughout its lifecycle.
Ideally, NDA-level planning should begin by Phase 2b. Starting early helps reduce the risk of rework and regulatory delays by allowing time for validation, data collection, and development of a robust control strategy.
Yes, the IND formulation can be used at the NDA stage if it’s supported with appropriate stability and bioavailability data. If significant changes occur, bridging studies may be required to confirm equivalency.
No, method validation is not mandatory at the IND stage. However, the methods must be scientifically qualified to ensure they are suitable for clinical use. Full validation is required before NDA submission.
For IND submissions, companies typically provide at least three months of accelerated or real-time stability data. This data must support the proposed shelf life during the clinical trial period and ensure patient safety.
Reference
- U.S. Food and Drug Administration. (2024, November 19). Chemistry manufacturing and controls (CMC) guidances for industry (GFIs) and questions and answers (Q&As). U.S. Department of Health and Human Services. https://www.fda.gov/animal-veterinary/guidance-industry/chemistry-manufacturing-and-controls-cmc-guidances-industry-gfis-and-questions-and-answers-qas
- Popkin, M. E., Goese, M., Wilkinson, D., Finnie, S., Flanagan, T., Campa, C., Clinch, A., Teasdale, A., Lennard, A., Cook, G., & Mohan, G. (2022). Chemistry manufacturing and controls development, industry reflections on manufacture, and supply of pandemic therapies and vaccines. AAPS Journal, 24(6), Article 101. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9514697/