Summary of Key Insights
- Integrated Chemistry and Analytical Services accelerate early drug discovery by combining chemical synthesis, bioassay development, and analytical validation into one continuous workflow.
- Early integration reduces cycle times, minimizes rework, and ensures data integrity from hit identification to lead optimization.
- Analytical support ensures compound purity, stability, and structure confirmation — critical for reliable pharmacological evaluations.
- AI-driven data integration and advanced LC-MS technologies now enhance compound profiling, accelerating candidate progression.
- Collaboration between medicinal chemists and analytical scientists improves decision-making and derisks development programs.
- Strategic partnerships with specialized labs like ResolveMass ensure access to state-of-the-art instrumentation and expertise across multiple therapeutic modalities.
Introduction: Why Integrated Chemistry and Analytical Services Are Essential in Early Drug Discovery
In early drug discovery, Integrated Chemistry and Analytical Services form the foundation for moving smoothly from molecular ideas to validated drug candidates. These integrated workflows allow chemists and analytical experts to work as one coordinated team. Every synthesized compound is carefully tested for identity, purity, and quality before moving forward. This ensures scientific accuracy from the very first experiment.
By removing traditional data silos, integration enables faster and smarter decisions. Analytical results are delivered quickly and used to guide chemical optimization. This reduces uncertainty and avoids delays caused by disconnected processes. In modern pharmaceutical research, speed and data quality must go hand in hand.
Unlike silo-based models, integrated systems create a continuous flow from synthesis to validation. Each step builds on the previous one, creating a strong and reliable discovery pipeline. This structure improves efficiency while maintaining high scientific standards. Ultimately, it helps programs advance with confidence.
Streamline your path from concept to candidate with specialized chemistry support designed for rapid innovation. Explore our Custom Synthesis Services for Startups
1. The Strategic Importance of Integrated Chemistry and Analytical Services in Discovery Pipelines
Integrated Chemistry and Analytical Services create a strong feedback loop between compound design, synthesis, and evaluation. Medicinal chemists receive analytical data almost immediately after synthesis. This allows rapid improvement of molecular structures based on real experimental results. Faster feedback leads to better compound design.
Embedding analytical support directly into chemistry workflows helps identify problems early. Issues such as low purity, incorrect structure, or poor yield can be corrected before biological testing begins. This prevents wasted time and unreliable assay outcomes. Early problem detection saves both cost and effort.
A unified chemistry and analytics framework also improves collaboration across teams. Chemists, biologists, and data scientists work from the same information. Shared visibility improves strategic planning and decision-making. In the long run, integration increases the chances of discovery success.
Optimize your hit-to-lead journey with expert medicinal chemistry insights and integrated laboratory workflows. Discover our Medicinal Chemistry Services
Key Benefits of an Integrated Chemistry Framework
| Benefit | Description |
|---|---|
| Accelerated Cycle Times | Rapid analytical turnaround enables faster hit-to-lead progression. |
| Reduced Redundancy | Integrated systems prevent repetitive compound characterization and reanalysis. |
| Improved Reproducibility | Consistent methods and shared datasets enhance data integrity across teams. |
| Higher Success Rates | Early detection of synthetic or analytical issues reduces late-stage attrition. |
For example, when chemists receive LC-MS or NMR data within hours, they can make immediate structure-activity decisions. This saves weeks of repeated experiments. Over time, these time savings significantly shorten development timelines. The impact grows across the entire program.
2. Analytical Support: A Core Element of Integrated Chemistry and Analytical Services
Analytical chemistry is not just a confirmation step; it is a critical decision-making tool. Each compound must meet defined quality standards before biological evaluation. Integrated Chemistry and Analytical Services ensure these standards are met consistently. This reliability supports all downstream research.
Without proper analytical validation, biological data can be misleading. Impurities or incorrect structures may distort assay results. Integrated analytics identify these issues early. This protects the accuracy and reproducibility of pharmacological studies.
Analytical data also support regulatory documentation and intellectual property protection. Well-organized records strengthen patent filings and future submissions. Integration ensures that data remains accessible and standardized. This makes discovery programs both scientifically and legally strong.
Core Analytical Capabilities Supporting Early Discovery
- LC-MS/MS profiling for molecular weight confirmation and impurity detection
- NMR spectroscopy for structure and stereochemistry verification
- Chromatographic purity analysis (HPLC, UPLC) for reliable bioassays
- Stability and degradation studies to assess compound robustness
- Mass-directed purification for efficient lead isolation
When these tools are fully integrated, every decision is supported by validated data. Results are traceable, reproducible, and trustworthy. This builds confidence throughout the discovery process. It also reduces the risk of late-stage failures.
Ensure the highest level of data integrity with rigorous method development tailored to your novel compounds. Learn about Custom Analytical Method Development
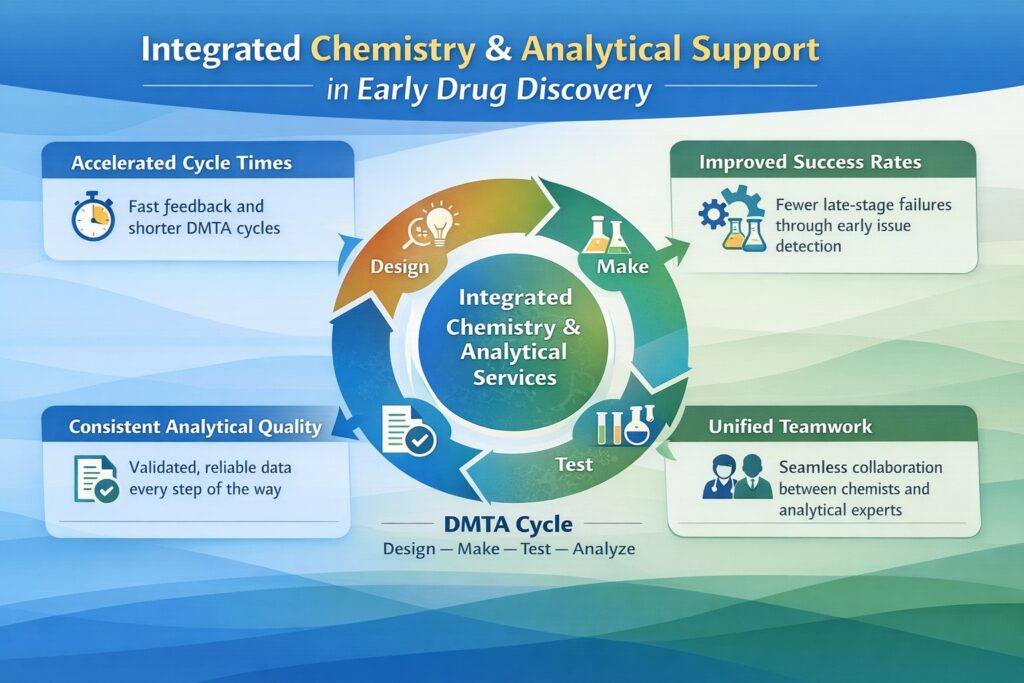
3. Integrated Chemistry and Analytical Services in the DMTA Cycle
The Design–Make–Test–Analyze (DMTA) cycle is central to medicinal chemistry. Integrated Chemistry and Analytical Services significantly shorten this cycle. What once took weeks can now be completed in days. Speed is crucial in competitive discovery environments.
Analytical feedback immediately informs design choices. Chemists do not need to wait for external validation. This quick response improves learning efficiency and compound optimization. Faster cycles mean faster progress.
Integrated DMTA workflows also support computational and predictive modeling. Analytical data feeds directly into these systems. Each cycle becomes smarter and more adaptive. This leads to higher-quality leads.
Workflow Example: Integrated DMTA Cycle
- Design – Medicinal chemists propose new structures using SAR insights
- Make – Compounds are synthesized using efficient laboratory systems
- Test – Analytical tools confirm purity and identity
- Analyze – Data informs the next optimization step
This closed-loop system improves learning with every cycle. AI-enabled tools further enhance accuracy. Discovery programs maintain momentum without sacrificing quality.
Accelerate your research timelines by partnering with a CRO that prioritizes speed without compromising scientific rigor. View our CRO Chemistry Service Timelines
4. Advanced Technologies Supporting Integrated Chemistry and Analytical Services
New analytical technologies have transformed early drug discovery. Modern instruments provide faster, more sensitive, and more accurate results. Integrated Chemistry and Analytical Services ensure these tools are fully utilized. This is essential for complex compound libraries.
Advanced platforms detect low-level impurities and metabolites early. This improves safety and stability assessment. Early insights reduce long-term risk. Integration ensures these benefits are realized quickly.
Automation and digital processing reduce manual work. Scientists can focus on interpretation rather than data handling. Productivity and data quality both improve. This leads to better scientific outcomes.
Technologies Empowering Integration
- High-resolution mass spectrometry for impurity and metabolite analysis
- Advanced NMR techniques for fast structure confirmation
- UPLC systems for precise purity profiling
- AI-based data processing for faster interpretation
These technologies reduce turnaround times and improve confidence in results. Early validation becomes more efficient. The entire discovery pipeline benefits.
Get precise insights into metabolic pathways with high-purity metabolites synthesized for your specific study needs. Explore Metabolite Synthesis Services
5. Collaborative Models in Integrated Chemistry and Analytical Services
Integration encourages continuous collaboration between chemists and analytical scientists. Communication becomes part of daily operations rather than a final step. Shared platforms keep everyone aligned. This reduces misunderstandings and delays.
Centralized data systems ensure transparency and consistency. Analytical priorities match chemistry goals. Teams work more efficiently. This coordination supports faster progress.
Collaboration also improves regulatory readiness. Standardized reporting simplifies audits and reviews. Data quality becomes a shared responsibility. This culture supports long-term research success.
Benefits of Cross-Functional Collaboration
- Better traceability from synthesis to characterization
- Faster analytical prioritization
- Unified reporting systems
- Improved resource utilization
Scale your research capabilities instantly by integrating an expert external team into your existing workflow. Discover our Virtual Chemistry Department Solutions
6. Data Integrity and Compliance in Integrated Chemistry and Analytical Services
Data integrity is a major advantage of integrated systems. These platforms follow ALCOA+ principles to ensure accuracy and traceability. Every data point is recorded correctly. This builds scientific credibility.
Centralized systems reduce data loss and inconsistency. Audit trails track every analytical action. Version control maintains transparency. These features support long-term compliance.
Regulatory readiness is built into daily workflows. When programs advance, documentation is already prepared. This reduces delays and rework. Integrated systems support both science and compliance.
Secure your intellectual property with a chemistry partner committed to absolute confidentiality and robust documentation. Learn about our Outsourced Chemistry for Biotech
7. Integrated Chemistry and Analytical Services in Lead Optimization
During lead optimization, analytical insights directly guide chemistry decisions. LC-MS data can reveal metabolic weaknesses. Chemists can redesign molecules accordingly. Integration makes this process fast and effective.
Analytical results also help prioritize synthetic routes. High-purity pathways are favored. Risks are identified early. This saves time and resources.
Integrated data turns results into actionable knowledge. Decisions are proactive. Lead optimization becomes more strategic and efficient.
Achieve greater cost-efficiency in your discovery program by leveraging lean, high-impact synthetic strategies. Explore Cost-Effective Drug Discovery Chemistry
8. AI, Automation, and the Future of Integrated Chemistry and Analytical Services
AI and automation are reshaping integrated discovery workflows. Predictive tools guide synthesis and purification. Robotics improve consistency. Human expertise is enhanced, not replaced.
Machine learning uncovers hidden SAR patterns. Analytical and biological data combine into powerful insights. Discovery becomes smarter and faster.
Cloud-based platforms connect global teams. Integration scales easily. The future laboratory is digital, connected, and efficient.
Conclusion: Why Integrated Chemistry and Analytical Services Matter
In today’s drug discovery landscape, Integrated Chemistry and Analytical Services are essential. They unify synthesis, validation, and analytics into one efficient system. Decisions become faster and more reliable. Compliance is built in from the start.
ResolveMass Laboratories Inc. demonstrates this integrated model through advanced tools and expert teams. Their approach supports partners from early discovery through lead optimization. Integration becomes a true competitive advantage.
For tailored support in integrated chemistry and analytical solutions, contact our expert team today:
👉 Contact us here
Frequently Asked Questions (FAQs)
Integrated chemistry and analytical support ensures that every compound is properly identified, purified, and verified before biological testing. This reduces the risk of misleading results and avoids repeating experiments. It also helps teams make faster and more confident decisions. Overall, it improves both speed and data reliability.
AI helps analyze large volumes of chemical and analytical data quickly and accurately. It can identify patterns, predict purification outcomes, and support compound design decisions. This reduces manual effort and human error. As a result, discovery teams work more efficiently.
Accurate and well-documented data ensures that results can be trusted and reproduced. Poor data quality can lead to wrong conclusions and costly delays. Integrated systems help maintain clear records and audit trails. This also supports future regulatory and patent requirements.
Non-integrated workflows often cause delays due to poor communication between teams. Compounds may be tested before proper validation, leading to unreliable data. Repeated testing and data inconsistencies are also common. These issues slow down the entire discovery process.
Yes, modern integrated platforms can handle small molecules, peptides, and other complex compounds. Analytical methods are adapted based on the molecule type. This flexibility allows teams to work across multiple therapeutic areas. It also supports diverse research pipelines.
Reference
- BioSolveIT GmbH. (n.d.). CROs for drug discovery: Partners for research. BioSolveIT. Retrieved January 13, 2026, from https://www.biosolveit.de/drug-discovery-solutions/cros-for-drug-discovery/
- Steadman, V. A. (2018). Drug discovery: Collaborations between contract research organizations and the pharmaceutical industry. ACS Medicinal Chemistry Letters, 9(7), 581–583. https://doi.org/10.1021/acsmedchemlett.8b00236