
Introduction
Poly(β-amino esters): Structure and Properties represent a critical area of research in modern polymer science, particularly in pharmaceutical and biomedical applications. Poly(β-amino esters), often abbreviated as PBAEs, are synthetic, biodegradable polymers known for their tunable chemistry, cationic nature, and excellent compatibility with biological systems.
Poly(β-amino esters) (PBAEs) have emerged as a promising class of biodegradable polymers, gaining attention in drug delivery systems due to their unique properties. Known for their versatility, ease of synthesis, and tunable characteristics, PBAEs stand out among polymers for their potential in delivering therapeutic agents, particularly nucleic acids like mRNA, siRNA, and plasmid DNA. This article provides a comprehensive introduction to Poly(β-amino esters), focusing on their structure, properties, and applications in biomedical research.
To understand how PBAEs get their unique structure, the guide on RAFT and ATRP is a great starting point.
Summary
- Poly(β-amino esters) are biodegradable, cationic polymers widely explored for drug delivery and gene delivery applications.
- Their chemical structure directly controls properties such as degradability, solubility, charge density, and biocompatibility.
- Poly(β-amino esters): Structure and Properties can be precisely tuned through monomer selection and polymerization conditions.
- These polymers offer excellent versatility for advanced pharmaceutical and biomedical research.
1. What are Poly(β-amino esters)?
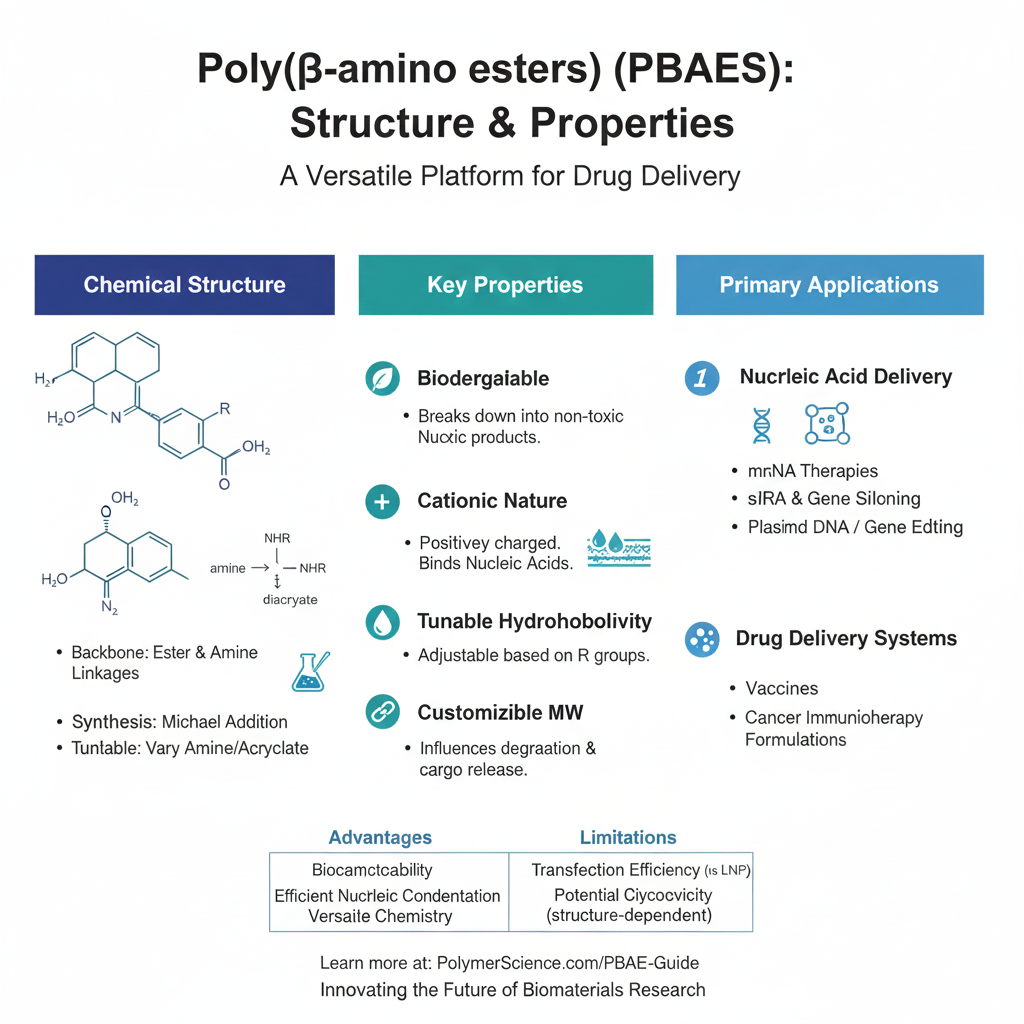
Poly(β-amino esters) are linear or branched polymers composed of repeating units with ester and amine linkages. The general structure includes a backbone derived from the Michael addition reaction between primary or secondary amines and diacrylates. These polymers are often synthesized through a straightforward process that allows researchers to easily modify their structure by changing the amines or acrylates used in the reaction.
The ability to introduce functional groups into the polymer backbone allows for fine-tuning of its physical and chemical properties, which can be customized based on the intended application. This tunability is particularly beneficial for optimizing their use in drug delivery systems, where factors like solubility, biodegradability, and mechanical properties play critical roles [1,2].
Understanding custom polymer synthesis gives better context to how PBAEs are developed.
1.1 Key Characteristics of Poly(β-amino esters)
- Biodegradability: PBAEs degrade into non-toxic byproducts in physiological conditions, making them suitable for clinical applications [3].
- Cationic Nature: The presence of amino groups imparts a positive charge, enabling electrostatic interaction with negatively charged molecules like nucleic acids.
- Tunable Hydrophobicity: By altering the polymer’s structure, PBAEs can be made more hydrophobic or hydrophilic depending on the delivery system’s requirements.
- Customizable Molecular Weight: Researchers can control the molecular weight during synthesis, which influences the polymer’s ability to encapsulate drugs and its degradation rate.
PBAEs aren’t just unique, they’re part of a bigger trend in designing smarter, more eco-friendly materials. Learn more here – Emerging Trends in Custom Polymer Synthesis for 2025 and Beyond
Chemical Structure of Poly(β-amino esters)
The structure of Poly(β-amino esters) directly determines their physical, chemical, and biological properties. At the molecular level, PBAEs consist of repeating units formed by Michael addition reactions.
Core Structural Components
| Structural Element | Role in Polymer Properties |
|---|---|
| β-amino ester linkage | Enables biodegradability |
| Tertiary amines | Provide cationic charge |
| Aliphatic spacers | Control flexibility and hydrophobicity |
| End-group chemistry | Influences stability and cell interaction |
Understanding Poly(β-amino esters): Structure and Properties requires close examination of how these components interact.
2. Applications in Drug Delivery Systems
The unique combination of properties makes Poly(β-amino esters) particularly useful in drug delivery systems, especially for nucleic acid delivery such as mRNA-based therapies, gene editing, and cancer immunotherapies. Their biodegradable nature and cationic charge allow them to form stable complexes with nucleic acids, protecting the therapeutic cargo from degradation while promoting efficient cellular uptake [4].
2.1 mRNA Delivery
The use of PBAEs in mRNA delivery has garnered significant interest, particularly in the development of vaccines and gene therapies. Their ability to form nanoparticles that encapsulate and protect mRNA during delivery has been demonstrated in several studies, where PBAEs achieved efficient transfection and expression of therapeutic proteins [5,6].
3. Synthesis of Poly(β-amino esters)
The synthesis of Poly(β-amino esters) is generally performed through the Michael addition reaction, which involves reacting a primary or secondary amine with a diacrylate. This reaction results in the formation of ester linkages and introduces amine groups into the polymer chain. By selecting different amines and diacrylates, researchers can tailor the polymer’s molecular weight, charge density, and hydrophobicity [7].
The simplicity of the synthesis process also allows for large-scale production, which is crucial for commercial and clinical applications.
Want a clear picture of how custom polymers are synthesized? Read this – Step-by-Step Guide to Custom Polymer Synthesis Process
4. Structure-Property Relationships
Understanding the structure-property relationships of Poly(β-amino esters) is crucial for their effective application in biomedical research. Several studies have shown that minor modifications in the polymer backbone, such as varying the length of alkyl chains or introducing cyclic structures, can significantly affect the polymer’s degradation rate, transfection efficiency, and toxicity profile [8].
For instance, PBAEs with longer hydrophobic chains tend to show slower degradation and more prolonged release of the encapsulated drug, which can be advantageous for sustained-release formulations. In contrast, shorter, more hydrophilic PBAEs may exhibit faster degradation and are more suitable for applications requiring rapid drug release.
The structure of a polymer starts with the monomer you pick – Monomer Selection Strategies for Custom Polymer Synthesis
5. Advantages and Limitations of Poly(β-amino esters)
5.1 Advantages:
- Biocompatibility: Poly(β-amino esters) degrade into non-toxic byproducts that are easily cleared from the body [3].
- Efficient Nucleic Acid Delivery: PBAEs efficiently condense nucleic acids into nanoparticles that protect them from degradation and facilitate their cellular uptake [5].
- Versatile Chemical Structure: The polymer backbone can be easily modified to adjust the polymer’s physical properties, such as molecular weight, charge density, and hydrophilicity [7].
These polymers have some great benefits, but also a few issues – Top Challenges and Opportunities in Custom Polymer Synthesis explains both in a simple way.
5.2 Limitations:
- Transfection Efficiency: While PBAEs have shown promise in nucleic acid delivery, their transfection efficiency may be lower compared to more established systems like lipid nanoparticles [6].
- Toxicity Concerns: Although PBAEs are generally biocompatible, certain structural modifications can lead to increased cytotoxicity, requiring careful consideration during polymer design [8].
Conclusion
Poly(β-amino esters) represent a versatile and promising material for drug delivery systems, especially in the realm of nucleic acid therapeutics. Their biodegradable nature, ability to be synthetically modified, and potential for safe and effective delivery make them a material of interest for future therapeutic applications. As research continues to advance, it is expected that Poly(β-amino esters) will play a critical role in the next generation of drug delivery platforms, particularly for mRNA therapies.
From properties to purpose – now explore how polymers like these are gaining traction in the market Global Market Insights: The Future of Custom Polymer Synthesis
Frequently Asked Questions:
The structure directly determines key properties such as degradation rate, solubility, molecular weight, and interaction with biological molecules. Small structural changes can significantly alter performance.
Poly(β-amino esters are typically synthesized using Michael addition polymerization between diacrylates and amines, allowing mild reaction conditions and excellent structural control.
Higher molecular weight generally improves delivery efficiency and stability, while lower molecular weight leads to faster degradation and reduced cytotoxicity.
They are characterized using techniques such as GPC for molecular weight, NMR for structural confirmation, mass spectrometry, and thermal analysis to validate structure–property relationships.
References
- Lynn, D. M., & Langer, R. Poly(β-amino esters): Synthesis, Properties, and Applications in Gene Delivery. J Am Chem Soc (2000). DOI: 10.1021/ja001012z.
- Green, J. J., et al. Synthesis and Biological Evaluation of Poly(β-amino esters) for Nucleic Acid Delivery. Adv Drug Deliv Rev (2007). DOI: 10.1016/j.addr.2006.10.008.
- Anderson, D. G., et al. Poly(β-amino esters) for Drug Delivery: Biocompatibility and Degradation. Biomaterials (2003). DOI: 10.1016/S0142-9612(03)00237-2.
- Zugates, G. T., et al. Poly(β-amino esters) for Gene Delivery: In Vitro and In Vivo Evaluation. Mol Ther (2007). DOI: 10.1038/sj.mt.6300082.
- Akinc, A., et al. Poly(β-amino esters) for mRNA Vaccine Delivery. Nat Biotechnol (2015). DOI: 10.1038/nbt.3071.
- Wang, Y., et al. Poly(β-amino esters) for Efficient Delivery of mRNA to Lung Cells. Adv Funct Mater (2020). DOI: 10.1002/adfm.201904145.
- Woodrow, K. A., et al. Poly(β-amino esters): Synthesis and Applications in Drug Delivery. Chem Rev (2008). DOI: 10.1021/cr0782067.
- Ou, M., et al. Poly(β-amino esters): Recent Advances and Challenges in Nucleic Acid Delivery. Biomacromolecules (2019). DOI: 10.1021/acs.biomac.8b01608.