Introduction
The increasing regulatory scrutiny on nitrosamine-related impurities has placed a strong emphasis on NDSRI Impurity Synthesis in Canada. Pharmaceutical companies, research institutions, and regulatory agencies require custom synthesis solutions to study, detect, and mitigate these impurities effectively. Choosing a specialized laboratory for NDSRI impurity synthesis in Canada ensures compliance with stringent regulatory requirements while accelerating drug development and impurity profiling.
In this guide, we explore the critical aspects of custom NDSRI impurity synthesis in Canada, its applications in pharmaceuticals and research, and how to select the right laboratory partner for impurity synthesis.
Understanding NDSRI Impurities
What Are NDSRIs?
Nitrosamine Drug Substance-Related Impurities (NDSRIs) are structurally linked to the active pharmaceutical ingredient (API) and can form during drug synthesis, storage, or degradation [1]. Due to their potential carcinogenic risk, regulatory agencies such as Health Canada, the FDA, and the EMA have imposed strict impurity limits.
Chemistry of NDSRIs
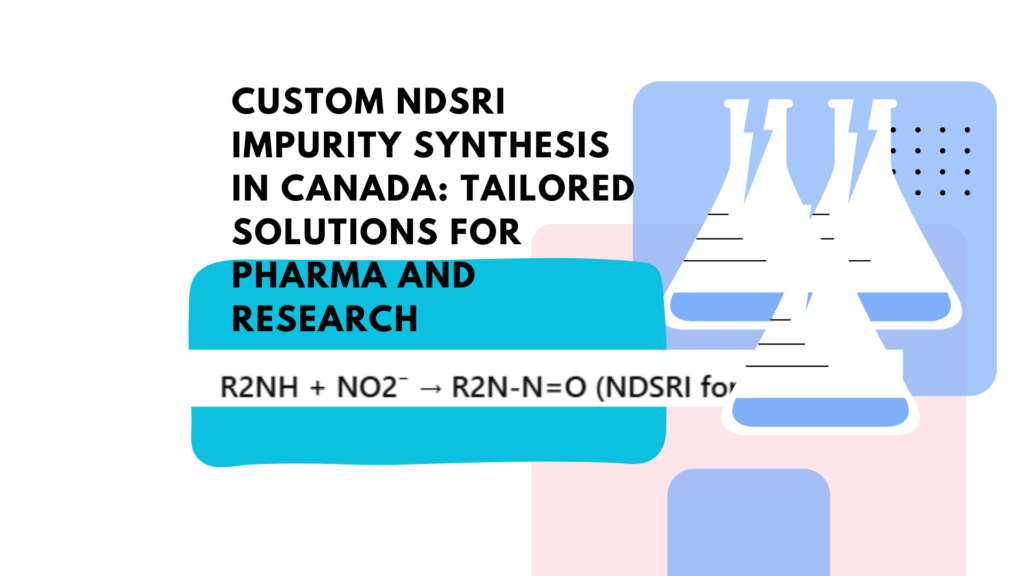
NDSRIs are formed when secondary or tertiary amines in drug molecules react with nitrosating agents such as nitrites under acidic conditions. This reaction follows the general pathway:
R2NH + NO2⁻ → R2N-N=O (NDSRI formation)
Several factors influence the formation of NDSRIs:
- pH Conditions: Acidic environments enhance nitrosation.
- Presence of Nitrites: Nitrites act as nitrosating agents.
- Molecular Structure: APIs with susceptible amine groups are at higher risk.
Examples of NDSRI Impurities and Their Structures
- N-Nitroso Propranolol
- Structure: Derived from propranolol (a beta-blocker), this NDSRI forms due to the presence of a secondary amine group.
- Formation Pathway: Occurs during storage when exposed to nitrosating conditions.
- N-Nitroso Varenicline
- Structure: Linked to varenicline, an anti-smoking medication.
- Impact: Detected in various lots, leading to product recalls due to regulatory concerns.
- N-Nitroso Atorvastatin
- Structure: A byproduct of atorvastatin (a cholesterol-lowering drug).
- Risk Factor: Forms due to manufacturing conditions, making impurity profiling essential.
Various Methods of NDSRI Impurity Synthesis in Pharmaceutical APIs
1. Direct Nitrosation of API Precursors
- Process: Secondary or tertiary amines in the drug molecule react with sodium nitrite in acidic conditions to form NDSRIs.
- Example: Synthesis of N-Nitroso Propranolol by reacting propranolol with sodium nitrite in hydrochloric acid.
- Application: Used to intentionally synthesize impurity standards for analytical validation.
2. Controlled Degradation Pathways
- Process: Degradation of the parent API under stress conditions (e.g., heat, humidity, acidic media) to study impurity formation.
- Example: N-Nitroso Metformin formation under acidic conditions.
- Application: Stability testing and forced degradation studies to predict impurity presence in formulations.
3. Metabolic Pathway Simulation
- Process: Mimicking in vivo metabolic transformations where nitrosation occurs in biological systems.
- Example: N-Nitroso Ranitidine formation through metabolic degradation in acidic gastric fluids.
- Application: Drug metabolism studies to understand impurity formation in human physiology.
4. Chemical Synthesis via Multi-Step Pathways
- Process: Using precursor molecules to construct NDSRIs through stepwise organic reactions.
- Example: Synthesis of N-Nitroso Atorvastatin using controlled oxidation and nitrosation reactions.
- Application: Producing high-purity NDSRI reference standards for regulatory testing.
5. Solid-State Nitrosation
- Process: Solid APIs interacting with trace nitrite impurities in excipients or packaging materials.
- Example: Formation of N-Nitroso Varenicline due to excipient interactions.
- Application: Investigating packaging and formulation factors contributing to impurity formation.
Why Custom NDSRI Impurity Synthesis in Canada is Essential
1. Regulatory Compliance and Risk Assessment
Health Canada has established stringent guidelines for the presence of nitrosamine-related impurities in pharmaceuticals [2]. To meet these requirements, companies must synthesize reference materials for accurate detection and risk assessment.
2. Supporting Pharmaceutical Research
- Impurity Profiling: Custom NDSRI synthesis allows for in-depth impurity profiling and characterization.
- Analytical Method Development: Tailored synthesis aids in validating testing methods for nitrosamine impurities.
- Drug Stability Studies: Understanding how NDSRIs form under various conditions ensures long-term drug safety.
3. Tailored Solutions for Unique API Structures
Unlike common nitrosamines (NDMA, NDEA), NDSRIs are API-specific. Laboratories offering custom NDSRI impurity synthesis in Canada design bespoke solutions to match the exact molecular structure of the API.
Custom NDSRI Impurity Synthesis: Key Considerations
1. Expertise in Nitrosamine Chemistry
Selecting a laboratory with expertise in nitrosamine formation pathways, degradation mechanisms, and analytical detection techniques is crucial [3].
2. Advanced Analytical Techniques
Reliable laboratories employ:
- LC-MS/MS (Liquid Chromatography-Mass Spectrometry)
- GC-MS/MS (Gas Chromatography-Mass Spectrometry)
- NMR Spectroscopy (Nuclear Magnetic Resonance)
3. Regulatory Accreditation
Ensure the lab complies with ISO/IEC 17025, Good Manufacturing Practices (GMP), and Health Canada guidelines.
Where to Get Custom NDSRI Impurity Synthesis in Canada
1. ResolveMass Laboratories – Your Trusted Partner
ResolveMass Laboratories is a leading provider of NDSRI impurity synthesis in Canada, offering:
- GMP-compliant, ISO/IEC 17025 accredited facilities
- Custom synthesis of NDSRI impurities tailored to specific APIs
- Regulatory-compliant impurity profiling and risk assessment
2. Case Studies: ResolveMass Expertise in NDSRI Impurity Synthesis
Case Study 1: Custom Synthesis of N-Nitroso Ranitidine Impurity
A pharmaceutical manufacturer needed a reference standard for N-Nitroso Ranitidine, a well-documented impurity in acid reflux medications. ResolveMass Laboratories developed a high-purity reference standard using:
- Multi-step synthetic pathways to match the impurity structure.
- Advanced LC-MS validation for accurate quantification.
The custom synthesis enabled the company to submit comprehensive impurity profiles to Health Canada, ensuring regulatory compliance and preventing market disruptions.
Case Study 2: Identification and Synthesis of N-Nitroso Metformin Impurity
A Canadian pharmaceutical firm faced a potential recall due to the detection of N-Nitroso Metformin. ResolveMass Laboratories provided a solution by:
- Synthesizing the impurity for method validation.
- Developing a risk assessment model to predict impurity formation in storage conditions.
The timely support helped the client avoid regulatory action and maintain product integrity.
3. Other Accredited Laboratories in Canada
While several Canadian laboratories offer impurity synthesis, businesses should verify their accreditation, expertise in NDSRI synthesis, and turnaround times.
How to Order Custom NDSRI Impurity Synthesis
Step 1: Contact an Accredited Lab
Reach out to a trusted provider like ResolveMass Laboratories to discuss synthesis requirements.
Step 2: Define Project Scope
Provide detailed information on the required impurity, including:
- Molecular structure
- Expected synthesis yield
- Purity and stability requirements
Step 3: Receive a Tailored Solution
The laboratory will design a synthesis pathway, conduct impurity profiling, and provide analytical reports.
Ensuring Regulatory Compliance with NDSRI Impurity Synthesis
Health Canada’s Guidelines on NDSRIs
Health Canada has set strict acceptable intake limits for nitrosamine impurities in pharmaceuticals [4]. Failure to comply may result in product recalls and regulatory sanctions.
How ResolveMass Helps You Stay Compliant
- Comprehensive impurity analysis reports
- Validated testing methods for regulatory submission
- Expert support in risk assessment and compliance
Conclusion
Choosing the right laboratory for NDSRI impurity synthesis in Canada is essential for ensuring regulatory compliance, drug safety, and research excellence. ResolveMass Laboratories offers tailored solutions for pharmaceutical companies and research institutions looking for reliable impurity synthesis. Contact us today to discuss your synthesis needs: Contact Us.
References
- EMA. (2021). Assessment and Mitigation of Nitrosamine Risk in Human Medicines. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf
- FDA. (2021). Control of Nitrosamine Impurities in Human Drugs. https://www.fda.gov/media/141720/download
- Health Canada. (2020). Guidance on Nitrosamine Impurities in Medications. https://www.canada.ca/en/health-canada/services/drugs-health-products.html
- ICH. (2023). ICH M7(R2) – Control of Mutagenic Impurities. https://database.ich.org/sites/default/files/M7_R2_Guideline_Step4_2023_0223.pdf

