Introduction
Nitrosamine Impurities Testing in Canada in pharmaceutical products have become a significant concern for regulatory bodies, including Health Canada and the U.S. FDA. The presence of these potentially carcinogenic compounds in medications necessitates thorough and reliable testing to ensure product safety and compliance with regulatory standards. For pharmaceutical manufacturers, contract research organizations (CROs), and healthcare institutions, selecting the right Nitrosamine Impurities Testing in Canada is crucial to maintaining compliance and ensuring public health. This guide will outline the essential factors to consider when choosing a testing provider, ensuring that your products meet the highest quality and regulatory standards.
Understanding Nitrosamine Impurities Testing in Canada
What Are Nitrosamines?
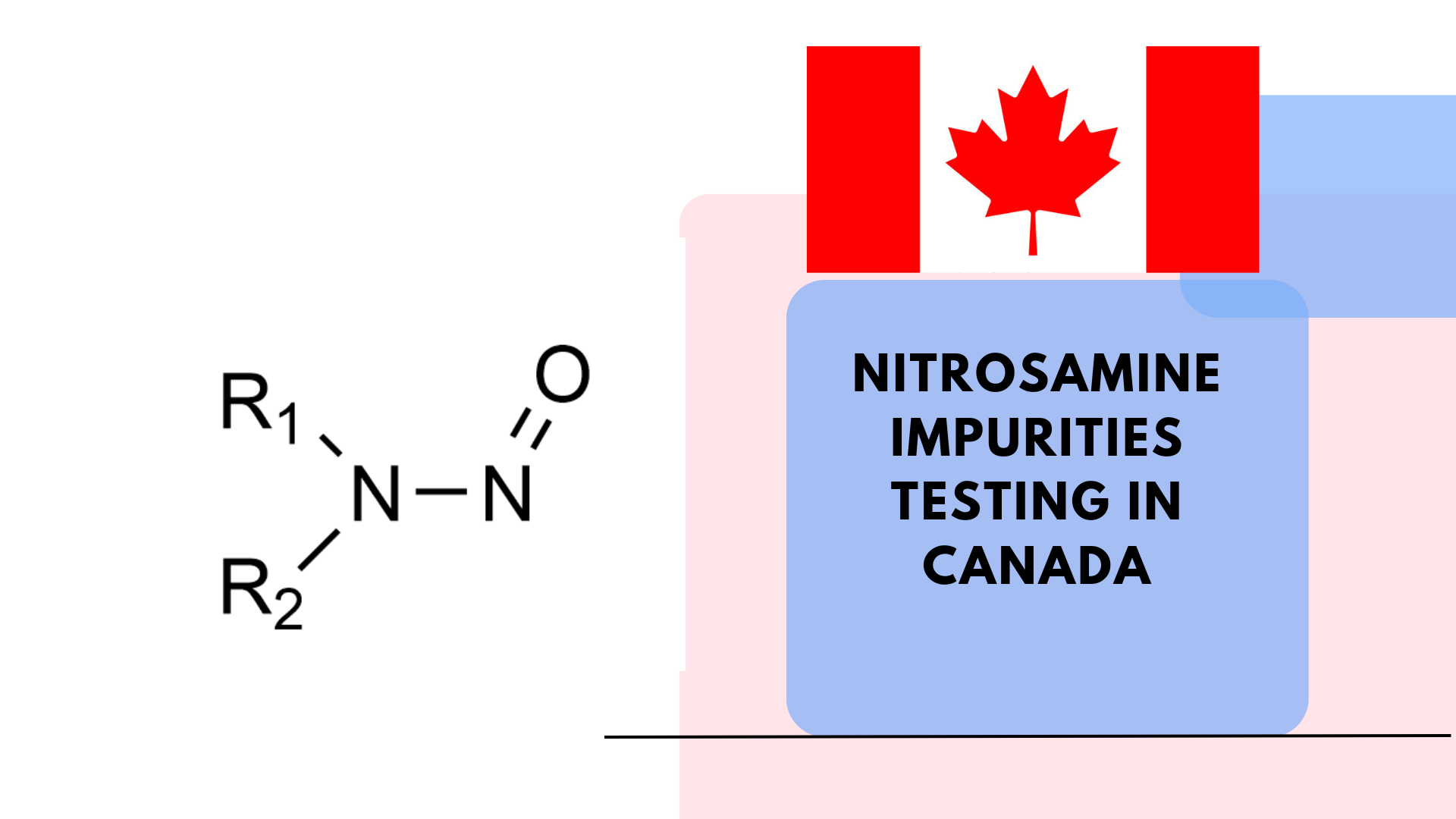
Nitrosamines are a class of chemical compounds classified as probable human carcinogens. They can form during pharmaceutical manufacturing processes due to reactions between nitrite and secondary amines. These impurities have been detected in various medications, leading to large-scale recalls and increased regulatory scrutiny [1]. Hence it is the most crucial aspects now to test Nitrosamine Impurities Testing in Canada.
Some common nitrosamines of concern include:
- N-nitrosodimethylamine (NDMA)
- N-nitrosodiethylamine (NDEA)
- N-nitroso-N-methyl-4-aminobutyric acid (NMBA)
Why Is Nitrosamine Impurities Testing in Canada Essential?
The significance of nitrosamine impurities testing cannot be overstated. Here’s why it is critical:
- Regulatory Compliance: Health Canada mandates rigorous testing for nitrosamines to prevent contamination in pharmaceuticals [2]. Failure to comply can lead to regulatory action, product recalls, and financial losses.
- Product Safety: Ensuring nitrosamine levels remain below permissible limits safeguards patients from potential health risks, including cancer.
- Market Access: Compliance with nitrosamine testing ensures uninterrupted product distribution in Canadian and global markets, preventing costly disruptions.
- Brand Reputation: Proactively addressing nitrosamine concerns builds consumer trust and prevents reputational damage that can arise from safety violations.
- Litigation Risks: Pharmaceutical companies that fail to test for nitrosamine impurities may face lawsuits from patients and regulatory fines, impacting long-term sustainability.
Key Factors to Consider When Choosing a Nitrosamine Impurities Testing Provider in Canada
1. Accreditation & Compliance Standards
The first and most crucial factor when selecting a testing service is verifying accreditation. A reputable laboratory should hold certifications from recognized regulatory bodies such as:
- Health Canada GMP (Good Manufacturing Practices) Compliance
- ISO/IEC 17025:2017 Accreditation
- U.S. FDA & ICH Compliance
- European Medicines Agency (EMA) authorization for international compliance
Regulatory bodies require validated analytical methods to detect nitrosamines at trace levels, ensuring pharmaceutical manufacturers meet safety and efficacy standards.
ResolveMass Laboratories adheres to these stringent standards, ensuring comprehensive and compliant nitrosamine analysis. Learn more about our accredited testing services: Nitrosamine Analysis Services.
2. Advanced Analytical Techniques
Nitrosamine testing requires highly sensitive analytical techniques, including:
- Gas Chromatography-Mass Spectrometry (GC-MS): Ideal for volatile nitrosamines with high sensitivity and specificity.
- Liquid Chromatography-Mass Spectrometry (LC-MS): Effective for detecting a broad range of nitrosamines in complex pharmaceutical formulations.
- High-Resolution Mass Spectrometry (HRMS): Provides detailed structural identification of unknown impurities.
These methods offer superior detection sensitivity, ensuring compliance with Health Canada’s strict limits [3]. Always verify that your testing provider employs these state-of-the-art techniques.
3. Experience in Pharmaceutical Testing for Nitrosamine Impurities Testing in Canada
Partnering with a laboratory that has a proven track record in pharmaceutical analysis ensures accuracy and reliability. Experienced testing providers are adept at handling complex formulations, excipients, and active pharmaceutical ingredients (APIs), minimizing risks of false positives or false negatives.
Questions to Ask Your Testing Provider:
- How many years of experience do you have in nitrosamine testing?
- Have you worked with pharmaceutical companies of similar scale and needs?
- Can you provide case studies or references from satisfied clients?
4. Turnaround Time & Efficiency
In a fast-paced pharmaceutical industry, quick and accurate results are critical. Choose a testing provider that offers:
- Expedited testing services for urgent regulatory submissions
- Efficient sample processing without compromising accuracy
- Real-time tracking of sample progress
ResolveMass Laboratories provides fast and reliable nitrosamine testing, ensuring minimal disruptions to your product development timelines: Nitrosamine Analysis Services.
5. Comprehensive Testing Capabilities for Nitrosamine Impurities Testing in Canada
A well-rounded testing laboratory should offer a broad range of nitrosamine analysis services, including:
- Risk assessment and method development to determine potential contamination sources
- Batch release testing to confirm product compliance before distribution
- Stability studies to monitor nitrosamine levels over time
- Root cause analysis to identify and mitigate contamination sources
The ability to conduct end-to-end testing ensures that all aspects of nitrosamine contamination are thoroughly assessed [4].
6. Regulatory Guidance & Support
A reputable laboratory should not only provide testing services but also offer regulatory consulting to help businesses navigate compliance requirements. Services such as:
- Health Canada submission assistance
- Nitrosamine risk evaluation and mitigation strategies
- Customized testing solutions based on specific drug formulations
7. Cost-Effectiveness & Transparency
While cost should not be the sole determining factor, it is essential to consider a provider that offers transparent pricing without hidden fees. Ensure that your chosen laboratory provides:
- Competitive pricing aligned with industry standards
- Clear breakdown of testing costs upfront
- Flexible service options based on your specific testing needs
Why Choose ResolveMass Laboratories for Nitrosamine Impurities Testing in Canada?
At ResolveMass Laboratories, we have established ourselves as a leader in pharmaceutical analysis and impurity testing in Canada. With decades of experience, we specialize in: ✔ Advanced nitrosamine detection using state-of-the-art LC-MS, GC-MS, and HRMS technologies
✔ Serving major pharmaceutical companies, CROs, and healthcare organizations across Canada and beyond
✔ Strict compliance with Health Canada, FDA, and ICH guidelines
✔ A team of highly skilled analytical chemists and regulatory experts
✔ Customized testing solutions tailored to your pharmaceutical formulations
✔ Rapid turnaround times and emergency testing services for critical cases
✔ Full regulatory support, including Health Canada submission assistance
As a trusted partner in pharmaceutical safety, ResolveMass Laboratories provides unparalleled expertise and precision in nitrosamine impurity testing. Learn more about our services here: Nitrosamine Analysis Services.
Conclusion
Choosing the best Nitrosamine Impurities Testing service in Canada requires careful consideration of accreditation, analytical techniques, industry experience, efficiency, and regulatory support. A high-quality testing partner ensures product safety, compliance with Health Canada regulations, and smooth market access.
To safeguard your pharmaceutical products, partner with an accredited and reliable testing laboratory. Contact ResolveMass Laboratories today for expert guidance and high-precision nitrosamine analysis: Contact Us.
References
- Health Canada. (2020). Guidance on Nitrosamine Impurities in Medications. Retrieved from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities.html
- U.S. Food & Drug Administration (FDA). (2021). Control of Nitrosamine Impurities in Human Drugs – Guidance for Industry. Retrieved from: https://www.fda.gov/media/141720/download
- European Medicines Agency (EMA). (2021). Assessment and Mitigation of Nitrosamine Risk in Human Medicines. Retrieved from: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf
- ICH M7(R2). (2023). Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Retrieved from: https://database.ich.org/sites/default/files/M7_R2_Guideline_Step4_2023_0223.pdf
External Links for Additional Resources
To further improve SEO and provide valuable resources for readers, consider adding outbound links to:
- Health Canada’s official nitrosamine guidelines: https://www.canada.ca/en/health-canada/services/drugs-health-products.html
- U.S. FDA’s latest updates on nitrosamine impurities: https://www.fda.gov/drugs
- EMA’s regulations on pharmaceutical impurities: https://www.ema.europa.eu/en