Why Nitrosamine Testing Acyclovir Is Important
With increasing global concern about nitrosamines in medicines, Nitrosamine Testing Acyclovir has become essential. Nitrosamines are harmful chemicals that may form during drug production or storage. They are considered possible cancer-causing agents. Though Acyclovir is widely used to treat herpes and shingles, its manufacturing process may involve chemicals and conditions that could lead to nitrosamine formation.
At ResolveMass Laboratories Inc., we help pharmaceutical companies detect and control nitrosamine risks in Acyclovir and similar drugs. Through our advanced testing and expert guidance, we ensure your products meet strict safety and regulatory requirements.
Explore our Nitrosamine Risk Assessment Guide »
Understanding Acyclovir and Its Manufacturing Process
How Acyclovir Is Made
Acyclovir is a synthetic antiviral medicine that mimics natural DNA building blocks. It contains a purine base and a flexible side chain. Although the final drug does not have harmful amine groups, the chemicals used during production could introduce nitrosamine risks.
The synthesis usually includes reacting guanine-based materials with chemicals like epichlorohydrin. Solvents such as DMF, DMA, and NMP are also used. These solvents can break down into compounds that may form nitrosamines, especially when mixed with nitrites or acids. Strict control over materials and conditions is key to preventing this.
General Synthesis of Acyclovir
The process of making Acyclovir usually starts with guanine or similar compounds. These are chemically modified using epichlorohydrin to add a hydroxyethoxymethyl side chain. After this reaction, the mixture goes through hydrolysis and is then purified to obtain the final nucleoside analog. Key factors like solvent choice, pH level, and temperature must be carefully controlled to improve yield and reduce unwanted impurities. Proper optimization at each stage helps lower the risk of forming harmful by-products, including nitrosamine precursors.
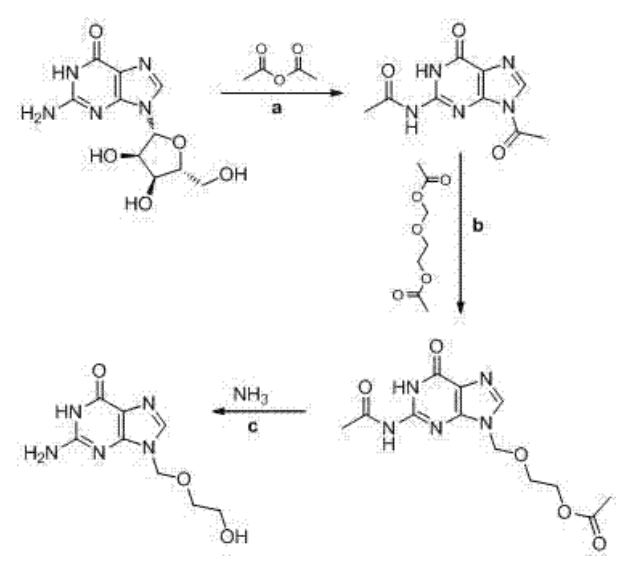
Zhang, J., Mai, W., Zhu, H., Wu, H., Xu, C., & Li, Y. (2014). Preparation method of acyclovir (CN103664944A). XINXIANG WEIDE CHEMICAL Co Ltd. https://patents.google.com/patent/CN103664944A/en
Nitrosamine Formation Risks in Acyclovir API
Nitrosamine risks in Acyclovir synthesis are linked to specific chemical steps. During silylation of guanine with HMDS, a primary amine at position 2 may form N-nitrosoguanine under acidic or nitrite-containing conditions, even though primary amines are generally less reactive.
The alkylation stage poses a greater risk. Polar solvents like DMF, DMA, or NMP, often used to aid solubility, can break down into DMA or DEA under heat or acidic conditions. If nitrites are present in raw materials or water, this may lead to the formation of NDMA or NDEA, both known carcinogenic nitrosamines.
During hydrolysis, when the acetal group is removed using acids such as acetic acid, N-nitrosoethylguanine could form, particularly if side chains degrade or reactions remain incomplete. Improperly purified recycled solvents further raise the risk by introducing residual amines or pre-formed nitrosamines.
Overall, nitrosamine formation in Acyclovir production is primarily associated with the silylation, alkylation, and hydrolysis stages.
Understand Nitrosamine CRO Support »
Nitrosamine Risks During Acyclovir Manufacturing
Nitrosamine formation during Acyclovir manufacturing can occur at specific points due to degradation, excipient interaction, and environmental factors.
While Acyclovir’s final API lacks secondary or tertiary amines, stress from heat, oxidation, or light during processes like milling or granulation may degrade the molecule, generating reactive amine fragments. These can react with nitrites in excipients to form nitrosamines such as N-nitrosoguanine or N-nitrosoethylguanine.
Common excipients like MCC, starch, lactose, and magnesium stearate often contain trace nitrites. Under humid or acidic conditions—such as in wet granulation or coating—these nitrites may react with amine impurities, potentially forming NDMA or related nitrosamines.
The use of recycled solvents or shared equipment, if not thoroughly cleaned, may introduce residual DMA or DEA, which can also react with formulation nitrites to form NDMA or NDEA during blending or compression.
In summary, nitrosamines in Acyclovir formulations may result from:
- API degradation under stress
- Nitrite impurities in excipients
- Residual amines from solvents or equipment
To reduce this risk, manufacturers must control excipient nitrite levels, avoid reactive solvent residues, and minimize heat, moisture, and low pH during processing.
Read Nitrosamine Testing in Apixaban »
Regulatory Status of Acyclovir-Related Nitrosamines
As of July 2025, there are no officially listed nitrosamines specifically linked to Acyclovir by Health Canada, the FDA, or the EMA. However, due to its structure, Acyclovir shares similarities with other nucleoside drugs that have shown nitrosamine risks.
Some potential impurities include:
- N-nitrosoguanine analogs
- N-nitrosoethylguanine (from the drug’s side chain)
- NDMA and NDEA (from solvent degradation)
These risks call for a proactive testing strategy, even if Acyclovir hasn’t been flagged directly.
See NDSRIs in Nitrosamine Testing »
Summary: Key Findings from Nitrosamine Testing Acyclovir
- Acyclovir is made using solvents and chemicals that can potentially form nitrosamines.
- The final drug is generally safe, but impurities and degradation products can increase risks.
- Recycled solvents and contaminated excipients can also add to the problem.
- No official nitrosamines for Acyclovir are listed yet, but it’s wise to stay ahead with regular testing.
Explore Nitrosamine Analysis Laboratory »
Why Choose ResolveMass for Nitrosamine Testing Acyclovir
Expert Solutions for Complete Nitrosamine Control
At ResolveMass Laboratories Inc., we specialize in Nitrosamine Testing Acyclovir using modern tools and expert knowledge. Here’s what we offer:
- Advanced detection tools like LC-MS/MS and GC-MS
- In-depth chemical and structural risk assessment
- Screening and control methods for potential NDSRIs
- Support for regulatory filings and global compliance
We guide pharmaceutical companies through the entire testing process — from early-stage risk analysis to finished product validation.
Start Your Risk Assessment Today »
FAQs
Nitrosamine testing for Acyclovir is important because nitrosamines are harmful chemicals that can form during drug production or storage. Some of these are linked to cancer, so checking for them helps protect patients. Testing also ensures the medicine meets safety rules set by health authorities.
Possible nitrosamines in Acyclovir include NDMA, NDEA, and N-nitrosoguanine. These may form from solvent breakdown, excipient reactions, or degradation during processing. Even small amounts of nitrites in excipients can lead to these impurities under certain conditions.
The finished Acyclovir API does not have secondary or tertiary amines, which are more likely to form nitrosamines. However, during production or storage, the drug can break down and create reactive amine-like parts that may react with nitrites to form nitrosamines.
NDSRIs (Nitrosamine Drug Substance Related Impurities) are found by studying the drug’s structure, how it’s made, and possible degradation. Scientists also use computer models, lab tests, and high-sensitivity tools like LC-MS or GC-MS to detect these impurities at very low levels.
Yes, testing each batch is important because small changes in materials, processes, or conditions can affect nitrosamine levels. Regular testing helps catch any problems early and ensures every batch of Acyclovir stays safe and within limits.
Contact ResolveMass for a customized testing strategy, including route evaluation, analytical setup, and regulatory documentation.
Contact us to begin your Acyclovir evaluation »
Conclusion: Your Trusted Partner for Nitrosamine Testing in Acyclovir
In a regulatory environment where safety and transparency are non-negotiable, nitrosamine testing for Acyclovir and its related APIs is essential. ResolveMass Laboratories Inc. offers unparalleled expertise in evaluating synthesis and manufacturing risks, detecting trace impurities, and maintaining regulatory compliance.
We empower pharmaceutical manufacturers to ensure drug safety and market access through robust nitrosamine control programs.
📌 Partner with ResolveMass today:
Contact Us Page 1
Contact Us Page 2
Contact Us Page 3
Internal Resources for Further Reading
- Nitrosamine Testing in Vonoprazan
- Acceptable Intake Nitrosamines: What You Need to Know
- Nitrosamine Testing in Betahistine and Related APIs
References
- Zhang, J., Mai, W., Zhu, H., Wu, H., Xu, C., & Li, Y. (2014). Preparation method of acyclovir (CN103664944A). XINXIANG WEIDE CHEMICAL Co Ltd. https://patents.google.com/patent/CN103664944A/en
- Control of Nitrosamine Impurities in Human Drugs